Isolation and characterization of olfactory ecto-mesenchymal stem cells from eight mammalian genera
- PMID: 29343270
- PMCID: PMC5772688
- DOI: 10.1186/s12917-018-1342-2
Isolation and characterization of olfactory ecto-mesenchymal stem cells from eight mammalian genera
Abstract
Background: Stem cell-based therapies are an attractive option to promote regeneration and repair defective tissues and organs. Thanks to their multipotency, high proliferation rate and the lack of major ethical limitations, "olfactory ecto-mesenchymal stem cells" (OE-MSCs) have been described as a promising candidate to treat a variety of damaged tissues. Easily accessible in the nasal cavity of most mammals, these cells are highly suitable for autologous cell-based therapies and do not face issues associated with other stem cells. However, their clinical use in humans and animals is limited due to a lack of preclinical studies on autologous transplantation and because no well-established methods currently exist to cultivate these cells. Here we evaluated the feasibility of collecting, purifying and amplifying OE-MSCs from different mammalian genera with the goal of promoting their interest in veterinary regenerative medicine. Biopsies of olfactory mucosa from eight mammalian genera (mouse, rat, rabbit, sheep, dog, horse, gray mouse lemur and macaque) were collected, using techniques derived from those previously used in humans and rats. The possibility of amplifying these cells and their stemness features and differentiation capability were then evaluated.
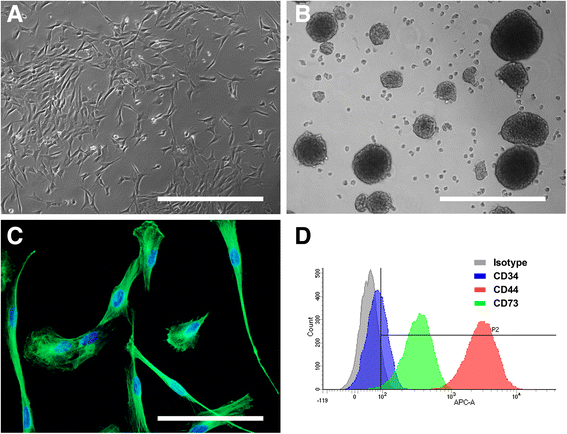
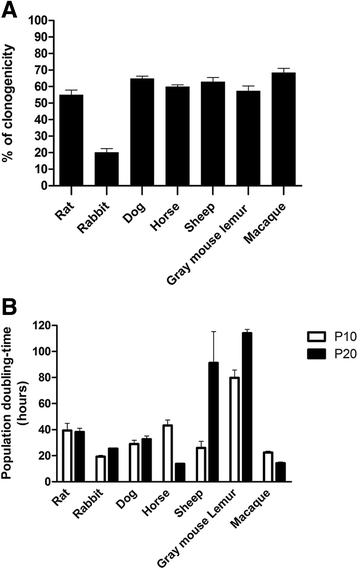
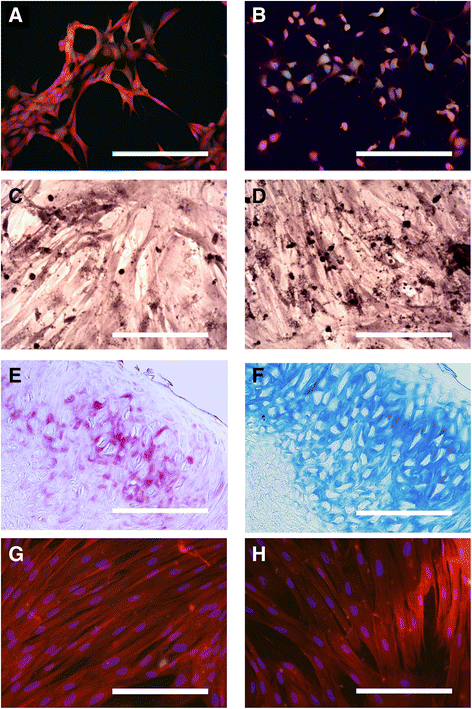
Results: Biopsies were successfully performed on olfactory mucosa without requiring the sacrifice of the donor animal, except mice. Cell populations were rapidly generated from olfactory mucosa explants. These cells displayed similar key features of their human counterparts: a fibroblastic morphology, a robust expression of nestin, an ability to form spheres and similar expression of surface markers (CD44, CD73). Moreover, most of them also exhibited high proliferation rates and clonogenicity with genus-specific properties. Finally, OE-MSCs also showed the ability to differentiate into mesodermal lineages.
Conclusions: This article describes for the first time how millions of OE-MSCs can be quickly and easily obtained from different mammalian genera through protocols that are well-suited for autologous transplantations. Moreover, their multipotency makes them relevant to evaluate therapeutic application in a wide variety of tissue injury models. This study paves the way for the development of new fundamental and clinical studies based on OE-MSCs transplantation and suggests their interest in veterinary medicine.
Keywords: Adult craniofacial stem cells; Dog; Ecto-mesenchymal stem cells; Horse; Non-human primate; Rabbit; Regenerative medicine; Rodent; Sheep; Veterinary medicine.
Conflict of interest statement
Ethics approval and consent to participate
Anesthesia and surgical procedures were performed according to the European law on Animal Care Guidelines and the Animal Care Committee of Aix-Marseille University and Ethic Committee of the Research Institute in Semiochemistry and Applied Ethology (C2EA125) approved our protocols. For client-owned animals, a written informed consent to participate was obtained through a specific authorization form.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Figures
Similar articles
-
Isolation and Characterization of Cat Olfactory Ecto-Mesenchymal Stem Cells.Animals (Basel). 2022 May 17;12(10):1284. doi: 10.3390/ani12101284. Animals (Basel). 2022. PMID: 35625130 Free PMC article.
-
Isolating nasal olfactory stem cells from rodents or humans.J Vis Exp. 2011 Aug 22;(54):2762. doi: 10.3791/2762. J Vis Exp. 2011. PMID: 21876529 Free PMC article.
-
A unique method for the isolation of nasal olfactory stem cells in living rats.Stem Cell Res. 2014 May;12(3):673-9. doi: 10.1016/j.scr.2014.02.010. Epub 2014 Mar 12. Stem Cell Res. 2014. PMID: 24681208
-
The therapeutic potential of human olfactory-derived stem cells.Histol Histopathol. 2006 Jun;21(6):633-43. doi: 10.14670/HH-21.633. Histol Histopathol. 2006. PMID: 16528674 Review.
-
Basic science and clinical application of stem cells in veterinary medicine.Adv Biochem Eng Biotechnol. 2010;123:219-63. doi: 10.1007/10_2010_66. Adv Biochem Eng Biotechnol. 2010. PMID: 20309674 Review.
Cited by
-
Human Olfactory Mesenchymal Stem Cells Are a Novel Candidate for Neurological Autoimmune Disease.Front Pharmacol. 2021 Dec 10;12:770884. doi: 10.3389/fphar.2021.770884. eCollection 2021. Front Pharmacol. 2021. PMID: 34955841 Free PMC article.
-
Exosomes as a New Delivery Vehicle in Inflammatory Bowel Disease.Pharmaceutics. 2021 Oct 9;13(10):1644. doi: 10.3390/pharmaceutics13101644. Pharmaceutics. 2021. PMID: 34683937 Free PMC article. Review.
-
Olfactory Ecto-Mesenchymal Stem Cells in Modeling and Treating Alzheimer's Disease.Int J Mol Sci. 2024 Aug 3;25(15):8492. doi: 10.3390/ijms25158492. Int J Mol Sci. 2024. PMID: 39126059 Free PMC article. Review.
-
Mesenchymal Stem Cells Isolated from Equine Hair Follicles Using a Method of Air-Liquid Interface.Stem Cell Rev Rep. 2023 Nov;19(8):2943-2956. doi: 10.1007/s12015-023-10619-w. Epub 2023 Sep 21. Stem Cell Rev Rep. 2023. PMID: 37733199 Free PMC article.
-
Isolation and Characterization of Neural Crest-Derived Stem Cells From Adult Ovine Palatal Tissue.Front Cell Dev Biol. 2018 Apr 11;6:39. doi: 10.3389/fcell.2018.00039. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 29696142 Free PMC article.
References
-
- Ribitsch I, Burk J, Delling U, et al. Basic science and clinical application of stem cells in veterinary medicine. Adv Biochem Eng Biotechnol. 2010;123:219–263. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

