Comparison of modified wet suction technique and dry suction technique in endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) for solid lesions: study protocol for a randomized controlled trial
- PMID: 29343303
- PMCID: PMC5773018
- DOI: 10.1186/s13063-017-2380-y
Comparison of modified wet suction technique and dry suction technique in endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) for solid lesions: study protocol for a randomized controlled trial
Abstract
Background: Several suction techniques have been developed recently to enhance tissue acquisition when sampling solid lesions using endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). The aim of this study is to determine whether a new modified wet suction technique (MWST) compared with the conventional dry suction technique (DRST) shall present better outcomes with respect to diagnostic yield and specimen quality of solid lesions in the intra-abdomen and mediastinum.
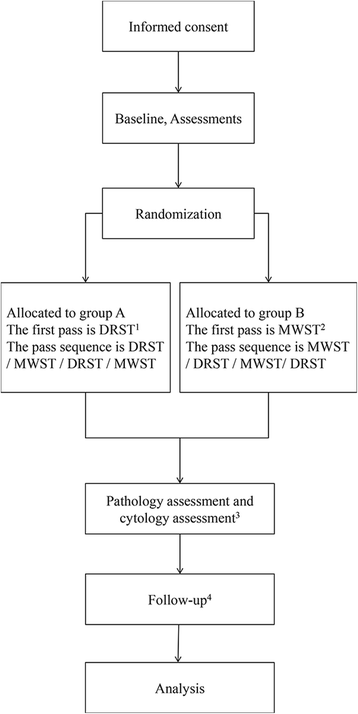
Methods/design: This is a single-blind, randomized, controlled, superiority trial conducted at four large tertiary care centers in China. Two hundred and ninety-six patients with solid lesions referred for EUS-FNA will be randomly assigned to group A, using DRST for the first pass, or group B, using MWST for the first pass in a ratio of 1:1. Following a 2 × 2 cross-over design, the pass sequence for group A is DRST, MWST, DRST, MWST. For group B, the pass sequence is MWST, DRST, MWST, DRST. All procedures will be performed by experienced echoendoscopists, and the patients and assessors (cytologists and pathologists) will be blinded during the entire study. The primary outcome measure is the diagnosis yield. Secondary outcome measures are specimen quality, including assessment of quantity of cell, tissue integrity, and blood contamination.
Discussion: To our knowledge, this is the first large-scale randomized controlled trial to compare MWST with DRST when sampling solid lesions in the intra-abdomen and mediastinum. The results may contribute to future multicenter clinical trials in standardizing suction techniques during EUS-FNA.
Trial registration: Clinical Trials.gov, NCT02789371 . Retrospectively registered on 6 June 2016.
Keywords: Diagnostic yield; Dry suction technique; EUS-FNA; Modified wet suction technique; Solid lesions.
Conflict of interest statement
Ethics approval and consent to participate
The study obtained ethics approval from the ethics committee of Tongji Medical College, Huazhong University of Science and Technology (IORG no., IORG0003571) on 4 May 2016. Written informed consent will be obtained from each participant or from each participant’s legally responsible relative prior to trial participation.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Polkowski M, Larghi A, Weynand B, Boustiere C, Giovannini M, Pujol B, Dumonceau JM, European Society of Gastrointestinal Endoscopy Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) technical guideline. Endoscopy. 2012;44(2):190–206. doi: 10.1055/s-0031-1291543. - DOI - PubMed
-
- Savides TJ, Donohue M, Hunt G, Al-Haddad M, Aslanian H, Ben-Menachem T, Chen VK, Coyle W, Deutsch J, DeWitt J, et al. EUS-guided FNA diagnostic yield of malignancy in solid pancreatic masses: A benchmark for quality performance measurement. Gastrointest Endosc. 2007;66(2):277–82. doi: 10.1016/j.gie.2007.01.017. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

