PAR1 biased signaling is required for activated protein C in vivo benefits in sepsis and stroke
- PMID: 29343482
- PMCID: PMC5855020
- DOI: 10.1182/blood-2017-10-810895
PAR1 biased signaling is required for activated protein C in vivo benefits in sepsis and stroke
Abstract
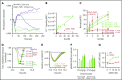
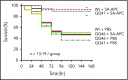
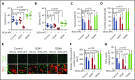
Activated protein C (APC) cleaves protease-activated receptor 1 (PAR1) in vitro at R46 to initiate beneficial cell signaling; however, thrombin and APC can cleave at R41. To elucidate PAR1-dependent aspects of the pharmacologic in vivo mechanisms of APC, we generated C57BL/6 mouse strains carrying QQ41 or QQ46 point mutations in PAR1 (F2r gene). Using these strains, we determined whether or not recombinant murine signaling-selective APC mutants would reduce septic death or provide neuroprotection against ischemic stroke when mice carried PAR1-homozygous mutations that prevent cleavage at either R41 or R46. Intercrossing PAR1+/R46Q mice generated expected numbers of PAR1+/+, PAR1+/R46Q, and R46Q/R46Q offspring whereas intercrossing PAR1+/R41Q mice gave decreased R41Q/R41Q homozygotes (resembling intercrossing PAR1+/PAR1-knockout mice). QQ41-PAR1 and QQ46-PAR1 brain endothelial cells showed the predicted retention or loss of cellular responses to thrombin receptor-activating peptide, thrombin, or APC for each PAR1 mutation. In sepsis studies, exogenous APC reduced mortality from 50% to 10% in Escherichia coli-induced pneumonia for wild-type (Wt) PAR1 and QQ41-PAR1 mice (P < .01) but had no benefit for QQ46-PAR1 mice. In transient distal middle cerebral artery occlusion stroke studies, exogenous APC significantly reduced infarct size, edema, and neuronal apoptosis for Wt mice and QQ41-PAR1 mice but had no detectable benefits for mice carrying QQ46-PAR1. In functional studies of forelimb-asymmetry and foot-fault tests at 24 hours after stroke induction, signaling-selective APC was beneficial for Wt and QQ41-PAR1 mice but not QQ46-PAR1 mice. These results support the concept that APC-induced, PAR1-dependent biased signaling following R46 cleavage is central to the in vivo benefits of APC.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: B.V.Z. is a founder of ZZ Biotech LLC, a biotechnology company with a mission to develop APC and its functional mutants for the treatment of stroke and other neurological disorders. L.O.M. and J.H.G. are inventors for some uses of APC mutants. J.H.G. is a consultant for ZZ Biotech LLC. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Cut me if you can!Blood. 2018 Mar 15;131(11):1155-1156. doi: 10.1182/blood-2018-02-829564. Blood. 2018. PMID: 29545401 No abstract available.
References
-
- Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64(6):1057-1068. - PubMed
-
- Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296(5574):1880-1882. - PubMed
-
- Cheng T, Liu D, Griffin JH, et al. . Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9(3):338-342. - PubMed
-
- Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109(8):3161-3172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

