A Population Phylogenetic View of Mitochondrial Heteroplasmy
- PMID: 29343499
- PMCID: PMC5844335
- DOI: 10.1534/genetics.118.300711
A Population Phylogenetic View of Mitochondrial Heteroplasmy
Abstract
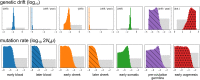
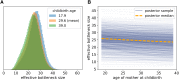
The mitochondrion has recently emerged as an active player in myriad cellular processes. Additionally, it was recently shown that >200 diseases are known to be linked to variants in mitochondrial DNA or in nuclear genes interacting with mitochondria. This has reinvigorated interest in its biology and population genetics. Mitochondrial heteroplasmy, or genotypic variation of mitochondria within an individual, is now understood to be common in humans and important in human health. However, it is still not possible to make quantitative predictions about the inheritance of heteroplasmy and its proliferation within the body, partly due to the lack of an appropriate model. Here, we present a population-genetic framework for modeling mitochondrial heteroplasmy as a process that occurs on an ontogenetic phylogeny, with genetic drift and mutation changing heteroplasmy frequencies during the various developmental processes represented in the phylogeny. Using this framework, we develop a Bayesian inference method for inferring rates of mitochondrial genetic drift and mutation at different stages of human life. Applying the method to previously published heteroplasmy frequency data, we demonstrate a severe effective germline bottleneck comprised of the cumulative genetic drift occurring between the divergence of germline and somatic cells in the mother, and the separation of germ layers in the offspring. Additionally, we find that the two somatic tissues we analyze here undergo tissue-specific bottlenecks during embryogenesis, less severe than the effective germline bottleneck, and that these somatic tissues experience little additional genetic drift during adulthood. We conclude with a discussion of possible extensions of the ontogenetic phylogeny framework and its possible applications to other ontogenetic processes in addition to mitochondrial heteroplasmy.
Keywords: cell lineage; development; phylogeny; somatic evolution.
Copyright © 2018 by the Genetics Society of America.
Figures



References
-
- Boisset J.-C., Robin C., 2012. On the origin of hematopoietic stem cells: progress and controversy. Stem Cell Res. 8: 1–13. - PubMed
-
- Cao L., Shitara H., Horii T., Nagao Y., Imai H., et al. , 2007. The mitochondrial bottleneck occurs without reduction of mtDNA content in female mouse germ cells. Nat. Genet. 39: 386–390. - PubMed
-
- Carling P. J., Cree L. M., Chinnery P. F., 2011. The implications of mitochondrial DNA copy number regulation during embryogenesis. Mitochondrion 11: 686–692. - PubMed
-
- Carlson B. M., 2014. Human Embryology and Developmental Biology, Ed. 5. Elsevier, Philadelphia.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

