Early and sustained efficacy with apremilast monotherapy in biological-naïve patients with psoriatic arthritis: a phase IIIB, randomised controlled trial (ACTIVE)
- PMID: 29343507
- PMCID: PMC5909747
- DOI: 10.1136/annrheumdis-2017-211568
Early and sustained efficacy with apremilast monotherapy in biological-naïve patients with psoriatic arthritis: a phase IIIB, randomised controlled trial (ACTIVE)
Abstract
Objective: Evaluate apremilast efficacy across various psoriatic arthritis (PsA) manifestations beginning at week 2 in biological-naïve patients with PsA.
Methods: Patients were randomised (1:1) to apremilast 30 mg twice daily or placebo. At week 16, patients whose swollen and tender joint counts had not improved by ≥10% were eligible for early escape. At week 24, all patients received apremilast through week 52.
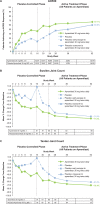
Results: Among 219 randomised patients (apremilast: n=110; placebo: n=109), a significantly greater American College of Rheumatology 20 response at week 16 (primary outcome) was observed with apremilast versus placebo (38.2% (42/110) vs 20.2% (22/109); P=0.004); response rates at week 2 (first assessment) were 16.4% (18/110) versus 6.4% (7/109) (P=0.025). Improvements in other efficacy outcomes, including 28-joint count Disease Activity Score (DAS-28) using C reactive protein (CRP), swollen joint count, Health Assessment Questionnaire-Disability Index (HAQ-DI), enthesitis and morning stiffness severity, were observed with apremilast at week 2. At week 16, apremilast significantly reduced PsA disease activity versus placebo, with changes in DAS-28 (CRP) (P<0.0001), HAQ-DI (P=0.023) and Gladman Enthesitis Index (P=0.001). Improvements were maintained with continued treatment through week 52. Over 52 weeks, apremilast's safety profile was consistent with prior phase 3 studies in psoriasis and PsA. During weeks 0-24, the incidence of protocol-defined diarrhoea was 11.0% (apremilast) and 8.3% (placebo); serious adverse event rates were 2.8% (apremilast) and 4.6% (placebo).
Conclusions: In biological-naïve patients with PsA, onset of effect with apremilast was observed at week 2 and continued through week 52. The safety profile was consistent with previous reports.
Trial registration number: NCT01925768; Results.
Keywords: Das28; disease activity; psoriatic arthritis; spondyloarthritis; treatment.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: PN has received grant/research support and honoraria from Celgene Corporation. KO has received grant/research support from Celgene Corporation. JW has received non-financial support from Amgen Inc, Pfizer and UCB and has received personal fees from Celgene Corporation and Novartis. ND, DN and LT are employees of Celgene Corporation. JJG-R has received grant/research support from Roche and Schering-Plough and has served as a consultant to Bristol-Myers Squibb, Pfizer, Roche, Schering-Plough and UCB. JAA has received grant/research support from AbbVie Inc, Ardea Biosciences, Inc, AstraZeneca, Bristol-Myers Squibb, Celgene Corporation, Centocor, Galápagos, Genentech Inc, GlaxoSmithKline, Human Genome Sciences, Janssen, Eli Lilly and Company, Merck & Co, Mesoblast, Novartis Pharmaceuticals Corporation, Novo Nordisk, Pfizer, Roche, Sanofi-Aventis, Takeda Pharmaceuticals, UCB and Vertex Pharmaceuticals.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

