TRPM2 channel-mediated regulation of autophagy maintains mitochondrial function and promotes gastric cancer cell survival via the JNK-signaling pathway
- PMID: 29343514
- PMCID: PMC5846146
- DOI: 10.1074/jbc.M117.817635
TRPM2 channel-mediated regulation of autophagy maintains mitochondrial function and promotes gastric cancer cell survival via the JNK-signaling pathway
Abstract
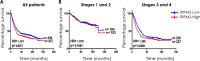
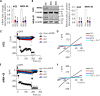
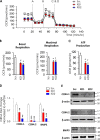
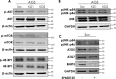
A lack of effective treatment is one of the main factors contributing to gastric cancer-related death. Discovering effective targets and understanding their underlying anti-cancer mechanism are key to achieving the best response to treatment and to limiting side effects. Although recent studies have shown that the cation channel transient receptor potential melastatin-2 (TRPM2) is crucial for cancer cell survival, the exact mechanism remains unclear, limiting its therapeutic potential. Here, using molecular and functional assays, we investigated the role of TRPM2 in survival of gastric cancer cells. Our results indicated that TRPM2 knockdown in AGS and MKN-45 cells decreases cell proliferation and enhances apoptosis. We also observed that the TRPM2 knockdown impairs mitochondrial metabolism, indicated by a decrease in basal and maximal mitochondrial oxygen consumption rates and ATP production. These mitochondrial defects coincided with a decrease in autophagy and mitophagy, indicated by reduced levels of autophagy- and mitophagy-associated proteins (i.e. ATGs, LC3A/B II, and BNIP3). Moreover, we found that TRPM2 modulates autophagy through a c-Jun N-terminal kinase (JNK)-dependent and mechanistic target of rapamycin-independent pathway. We conclude that in the absence of TRPM2, down-regulation of the JNK-signaling pathway impairs autophagy, ultimately causing the accumulation of damaged mitochondria and death of gastric cancer cells. Of note, by inhibiting cell proliferation and promoting apoptosis, the TRPM2 down-regulation enhanced the efficacy of paclitaxel and doxorubicin in gastric cancer cells. Collectively, we provide compelling evidence that TRPM2 inhibition may benefit therapeutic approaches for managing gastric cancer.
Keywords: TRPM2; autophagy; cell proliferation; chemotherapy; gastric cancer; mitochondria; transient receptor potential channels (TRP channels).
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

