A New Quinoline BRD4 Inhibitor Targets a Distinct Latent HIV-1 Reservoir for Reactivation from Other "Shock" Drugs
- PMID: 29343578
- PMCID: PMC5923069
- DOI: 10.1128/JVI.02056-17
A New Quinoline BRD4 Inhibitor Targets a Distinct Latent HIV-1 Reservoir for Reactivation from Other "Shock" Drugs
Abstract
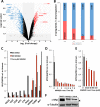
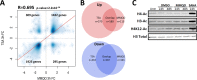
Upon HIV-1 infection, a reservoir of latently infected resting T cells prevents the eradication of the virus from patients. To achieve complete depletion, the existing virus-suppressing antiretroviral therapy must be combined with drugs that reactivate the dormant viruses. We previously described a novel chemical scaffold compound, MMQO (8-methoxy-6-methylquinolin-4-ol), that is able to reactivate viral transcription in several models of HIV latency, including J-Lat cells, through an unknown mechanism. MMQO potentiates the activity of known latency-reversing agents (LRAs) or "shock" drugs, such as protein kinase C (PKC) agonists or histone deacetylase (HDAC) inhibitors. Here, we demonstrate that MMQO activates HIV-1 independently of the Tat transactivator. Gene expression microarrays in Jurkat cells indicated that MMQO treatment results in robust immunosuppression, diminishes expression of c-Myc, and causes the dysregulation of acetylation-sensitive genes. These hallmarks indicated that MMQO mimics acetylated lysines of core histones and might function as a bromodomain and extraterminal domain protein family inhibitor (BETi). MMQO functionally mimics the effects of JQ1, a well-known BETi. We confirmed that MMQO interacts with the BET family protein BRD4. Utilizing MMQO and JQ1, we demonstrate how the inhibition of BRD4 targets a subset of latently integrated barcoded proviruses distinct from those targeted by HDAC inhibitors or PKC pathway agonists. Thus, the quinoline-based compound MMQO represents a new class of BET bromodomain inhibitors that, due to its minimalistic structure, holds promise for further optimization for increased affinity and specificity for distinct bromodomain family members and could potentially be of use against a variety of diseases, including HIV infection.IMPORTANCE The suggested "shock and kill" therapy aims to eradicate the latent functional proportion of HIV-1 proviruses in a patient. However, to this day, clinical studies investigating the "shocking" element of this strategy have proven it to be considerably more difficult than anticipated. While the proportion of intracellular viral RNA production and general plasma viral load have been shown to increase upon a shock regimen, the global viral reservoir remains unaffected, highlighting both the inefficiency of the treatments used and the gap in our understanding of viral reactivation in vivo Utilizing a new BRD4 inhibitor and barcoded HIV-1 minigenomes, we demonstrate that PKC pathway activators and HDAC and bromodomain inhibitors all target different subsets of proviral integration. Considering the fundamental differences of these compounds and the synergies displayed between them, we propose that the field should concentrate on investigating the development of combinatory shock cocktail therapies for improved reservoir reactivation.
Keywords: BETi; Brd4; HIV latency; LRAs.
Copyright © 2018 American Society for Microbiology.
Figures





References
-
- Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, Chomont N, Douek D, Lifson JD, Lo Y-R, Kuritzkes D, Margolis D, Mellors J, Persaud D, Tucker JD, Barre-Sinoussi F, International AIDS Society Towards a Cure Working Group, Alter G, Auerbach J, Autran B, Barouch DH, Behrens G, Cavazzana M, Chen Z, Cohen ÉA, Corbelli GM, Eholié S, Eyal N, Fidler S, Garcia L, Grossman C, Henderson G, Henrich TJ, Jefferys R, Kiem H-P, McCune J, Moodley K, Newman PA, Nijhuis M, Nsubuga MS, Ott M, Palmer S, Richman D, Saez-Cirion A, Sharp M, Siliciano J, Silvestri G, Singh J, Spire B, Taylor J, Tolstrup M, Valente S, van Lunzen J, Walensky R, Wilson I, Zack J. 2016. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 22:839–850. doi: 10.1038/nm.4108. - DOI - PMC - PubMed
-
- Hamer DH. 2004. Can HIV be cured? Mechanisms of HIV persistence and strategies to combat it. Curr HIV Res 2:99–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

