Polymorphic Human Sulfotransferase 2A1 Mediates the Formation of 25-Hydroxyvitamin D3-3- O-Sulfate, a Major Circulating Vitamin D Metabolite in Humans
- PMID: 29343609
- PMCID: PMC5829543
- DOI: 10.1124/dmd.117.078428
Polymorphic Human Sulfotransferase 2A1 Mediates the Formation of 25-Hydroxyvitamin D3-3- O-Sulfate, a Major Circulating Vitamin D Metabolite in Humans
Abstract
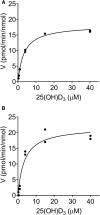
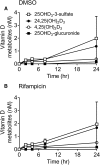
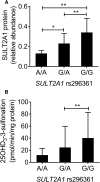
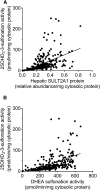
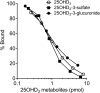
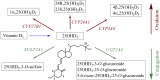
Metabolism of 25-hydroxyvitamin D3 (25OHD3) plays a central role in regulating the biologic effects of vitamin D in the body. Although cytochrome P450-dependent hydroxylation of 25OHD3 has been extensively investigated, limited information is available on the conjugation of 25OHD3 In this study, we report that 25OHD3 is selectively conjugated to 25OHD3-3-O-sulfate by human sulfotransferase 2A1 (SULT2A1) and that the liver is a primary site of metabolite formation. At a low (50 nM) concentration of 25OHD3, 25OHD3-3-O-sulfate was the most abundant metabolite, with an intrinsic clearance approximately 8-fold higher than the next most efficient metabolic route. In addition, 25OHD3 sulfonation was not inducible by the potent human pregnane X receptor agonist, rifampicin. The 25OHD3 sulfonation rates in a bank of 258 different human liver cytosols were highly variable but correlated with the rates of dehydroepiandrosterone sulfonation. Further analysis revealed a significant association between a common single nucleotide variant within intron 1 of SULT2A1 (rs296361; minor allele frequency = 15% in whites) and liver cytosolic SULT2A1 content as well as 25OHD3-3-O-sulfate formation rate, suggesting that variation in the SULT2A1 gene contributes importantly to interindividual differences in vitamin D homeostasis. Finally, 25OHD3-3-O-sulfate exhibited high affinity for the vitamin D binding protein and was detectable in human plasma and bile but not in urine samples. Thus, circulating concentrations of 25OHD3-3-O-sulfate appear to be protected from rapid renal elimination, raising the possibility that the sulfate metabolite may serve as a reservoir of 25OHD3 in vivo, and contribute indirectly to the biologic effects of vitamin D.
Copyright © 2018 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

