Keeping the home fires burning: AMP-activated protein kinase
- PMID: 29343628
- PMCID: PMC5805978
- DOI: 10.1098/rsif.2017.0774
Keeping the home fires burning: AMP-activated protein kinase
Abstract
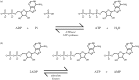
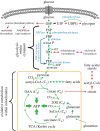
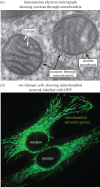
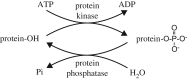
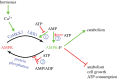
Living cells obtain energy either by oxidizing reduced compounds of organic or mineral origin or by absorbing light. Whichever energy source is used, some of the energy released is conserved by converting adenosine diphosphate (ADP) to adenosine triphosphate (ATP), which are analogous to the chemicals in a rechargeable battery. The energy released by the conversion of ATP back to ADP is used to drive most energy-requiring processes, including cell growth, cell division, communication and movement. It is clearly essential to life that the production and consumption of ATP are always maintained in balance, and the AMP-activated protein kinase (AMPK) is one of the key cellular regulatory systems that ensures this. In eukaryotic cells (cells with nuclei and other internal membrane-bound structures, including human cells), most ATP is produced in mitochondria, which are thought to have been derived by the engulfment of oxidative bacteria by a host cell not previously able to use molecular oxygen. AMPK is activated by increasing AMP or ADP (AMP being generated from ADP whenever ADP rises) coupled with falling ATP. Relatives of AMPK are found in essentially all eukaryotes, and it may have evolved to allow the host cell to monitor the output of the newly acquired mitochondria and step their ATP production up or down according to the demand. Structural studies have illuminated how AMPK achieves the task of detecting small changes in AMP and ADP, despite the presence of much higher concentrations of ATP. Recently, it has been shown that AMPK can also sense the availability of glucose, the primary carbon source for most eukaryotic cells, via a mechanism independent of changes in AMP or ADP. Once activated by energy imbalance or glucose lack, AMPK modifies many target proteins by transferring phosphate groups to them from ATP. By this means, numerous ATP-producing processes are switched on (including the production of new mitochondria) and ATP-consuming processes are switched off, thus restoring energy homeostasis. Drugs that modulate AMPK have great potential in the treatment of metabolic disorders such as obesity and Type 2 diabetes, and even cancer. Indeed, some existing drugs such as metformin and aspirin, which were derived from traditional herbal remedies, appear to work, in part, by activating AMPK.
Keywords: AMP-activated protein kinase; adenosine triphosphate; cell signalling; energy homeostasis; mitochondria.
© 2018 The Author(s).
Conflict of interest statement
I declare I have no competing interests.
Figures


References
-
- Hooke R. 1649. Micrographia. London, UK: The Royal Society.
-
- Ramaiah A, Hathaway JA, Atkinson DE. 1964. Adenylate as a metabolic regulator. Effect on yeast phosphofructokinase kinetics. J. Biol. Chem. 239, 3619–3622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
