Mechanogenetics for the remote and noninvasive control of cancer immunotherapy
- PMID: 29343642
- PMCID: PMC5798350
- DOI: 10.1073/pnas.1714900115
Mechanogenetics for the remote and noninvasive control of cancer immunotherapy
Abstract
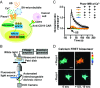
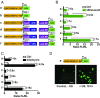
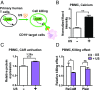
While cell-based immunotherapy, especially chimeric antigen receptor (CAR)-expressing T cells, is becoming a paradigm-shifting therapeutic approach for cancer treatment, there is a lack of general methods to remotely and noninvasively regulate genetics in live mammalian cells and animals for cancer immunotherapy within confined local tissue space. To address this limitation, we have identified a mechanically sensitive Piezo1 ion channel (mechanosensor) that is activatable by ultrasound stimulation and integrated it with engineered genetic circuits (genetic transducer) in live HEK293T cells to convert the ultrasound-activated Piezo1 into transcriptional activities. We have further engineered the Jurkat T-cell line and primary T cells (peripheral blood mononuclear cells) to remotely sense the ultrasound wave and transduce it into transcriptional activation for the CAR expression to recognize and eradicate target tumor cells. This approach is modular and can be extended for remote-controlled activation of different cell types with high spatiotemporal precision for therapeutic applications.
Keywords: cancer immunotherapy; mechanogenetics; remote control; synthetic biology; ultrasound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mechanogenetics for cellular engineering and cancer immunotherapy.Curr Opin Biotechnol. 2020 Dec;66:88-94. doi: 10.1016/j.copbio.2020.06.008. Epub 2020 Jul 24. Curr Opin Biotechnol. 2020. PMID: 32717634 Free PMC article. Review.
-
Inducible Gene Switches with Memory in Human T Cells for Cellular Immunotherapy.ACS Synth Biol. 2019 Aug 16;8(8):1744-1754. doi: 10.1021/acssynbio.8b00512. Epub 2019 Jul 16. ACS Synth Biol. 2019. PMID: 31268301 Free PMC article.
-
Cutting Edge: Piezo1 Mechanosensors Optimize Human T Cell Activation.J Immunol. 2018 Feb 15;200(4):1255-1260. doi: 10.4049/jimmunol.1701118. Epub 2018 Jan 12. J Immunol. 2018. PMID: 29330322
-
A self-inactivating retrovector incorporating the IL-2 promoter for activation-induced transgene expression in genetically engineered T-cells.Virol J. 2006 Nov 21;3:97. doi: 10.1186/1743-422X-3-97. Virol J. 2006. PMID: 17118192 Free PMC article.
-
Strategies for enhancing adoptive T-cell immunotherapy against solid tumors using engineered cytokine signaling and other modalities.Expert Opin Biol Ther. 2018 Jun;18(6):653-664. doi: 10.1080/14712598.2018.1473368. Epub 2018 May 14. Expert Opin Biol Ther. 2018. PMID: 29727246 Free PMC article. Review.
Cited by
-
Functional Expression of TRPV1 Ion Channel in the Canine Peripheral Blood Mononuclear Cells.Int J Mol Sci. 2021 Mar 20;22(6):3177. doi: 10.3390/ijms22063177. Int J Mol Sci. 2021. PMID: 33804707 Free PMC article.
-
Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours.Nat Rev Clin Oncol. 2023 Jan;20(1):49-62. doi: 10.1038/s41571-022-00704-3. Epub 2022 Nov 23. Nat Rev Clin Oncol. 2023. PMID: 36418477 Free PMC article. Review.
-
Emerging Piezo1 signaling in inflammation and atherosclerosis; a potential therapeutic target.Int J Biol Sci. 2022 Jan 1;18(3):923-941. doi: 10.7150/ijbs.63819. eCollection 2022. Int J Biol Sci. 2022. PMID: 35173527 Free PMC article. Review.
-
Activation of Piezo1 mechanosensitive ion channel in HEK293T cells by 30 MHz vertically deployed surface acoustic waves.Biochem Biophys Res Commun. 2019 Oct 20;518(3):541-547. doi: 10.1016/j.bbrc.2019.08.078. Epub 2019 Aug 23. Biochem Biophys Res Commun. 2019. PMID: 31451220 Free PMC article.
-
A mediator-free sonogenetic switch for therapeutic protein expression in mammalian cells.Nucleic Acids Res. 2025 Mar 20;53(6):gkaf191. doi: 10.1093/nar/gkaf191. Nucleic Acids Res. 2025. PMID: 40114374 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

