Cathelicidin-OA1, a novel antioxidant peptide identified from an amphibian, accelerates skin wound healing
- PMID: 29343843
- PMCID: PMC5772731
- DOI: 10.1038/s41598-018-19486-9
Cathelicidin-OA1, a novel antioxidant peptide identified from an amphibian, accelerates skin wound healing
Erratum in
-
Author Correction: Cathelicidin-OA1, a novel antioxidant peptide identified from an amphibian, accelerates skin wound healing.Sci Rep. 2018 Oct 23;8(1):15906. doi: 10.1038/s41598-018-33558-w. Sci Rep. 2018. PMID: 30349056 Free PMC article.
Abstract
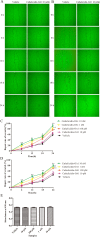
Cathelicidins play pivotal roles in host defense. The discovery of novel cathelicidins is important research; however, despite the identification of many cathelicidins in vertebrates, few have been reported in amphibians. Here we identified a novel cathelicidin (named cathelicidin-OA1) from the skin of an amphibian species, Odorrana andersonii. Produced by posttranslational processing of a 198-residue prepropeptide, cathelicidin-OA1 presented an amino acid sequence of 'IGRDPTWSHLAASCLKCIFDDLPKTHN' and a molecular mass of 3038.5 Da. Functional analysis showed that, unlike other cathelicidins, cathelicidin-OA1 demonstrated no direct microbe-killing, acute toxicity and hemolytic activity, but did exhibit antioxidant activity. Importantly, cathelicidin-OA1 accelerated wound healing against human keratinocytes (HaCaT) and skin fibroblasts (HSF) in both time- and dose-dependent manners. Notably, cathelicidin-OA1 also showed wound-healing promotion in a mouse model with full-thickness skin wounds, accelerating re-epithelialization and granulation tissue formation by enhancing the recruitment of macrophages to the wound site, inducing HaCaT cell proliferation and HSF cell migration. This is the first cathelicidin identified from an amphibian that shows potent wound-healing activity. These results will help in the development of new types of wound-healing agents and in our understanding of the biological functions of cathelicidins.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



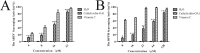



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

