Histone deacetylase 2 (HDAC2) attenuates lipopolysaccharide (LPS)-induced inflammation by regulating PAI-1 expression
- PMID: 29344006
- PMCID: PMC5763578
- DOI: 10.1186/s12950-018-0179-6
Histone deacetylase 2 (HDAC2) attenuates lipopolysaccharide (LPS)-induced inflammation by regulating PAI-1 expression
Abstract
Background: Sepsis is a life-threatening organ dysfunction caused by dysregulated host response to infection, and is primarily characterized by an uncontrolled systemic inflammatory response. In the present study, we developed an effective adjunct therapy mediated by a novel mechanism, to attenuate overt inflammation. LPS-treated macrophages were adopted as an in vitro model of endotoxin-induced inflammation during sepsis. Experiments were carried out using primary mouse peritoneal macrophages and the murine macrophage cell line RAW264.7, to elucidate the mechanisms by which HDAC2 modulates endotoxin-induced inflammation.
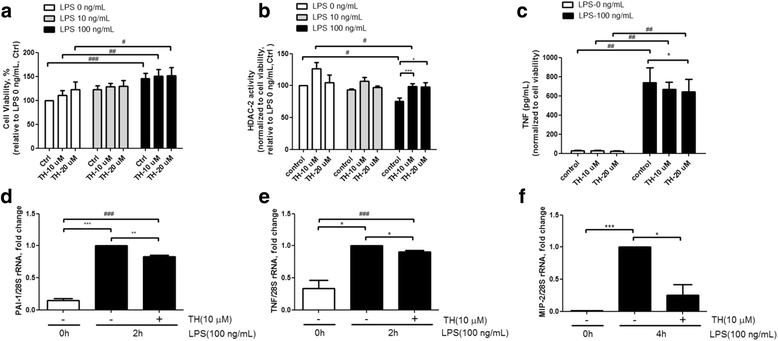
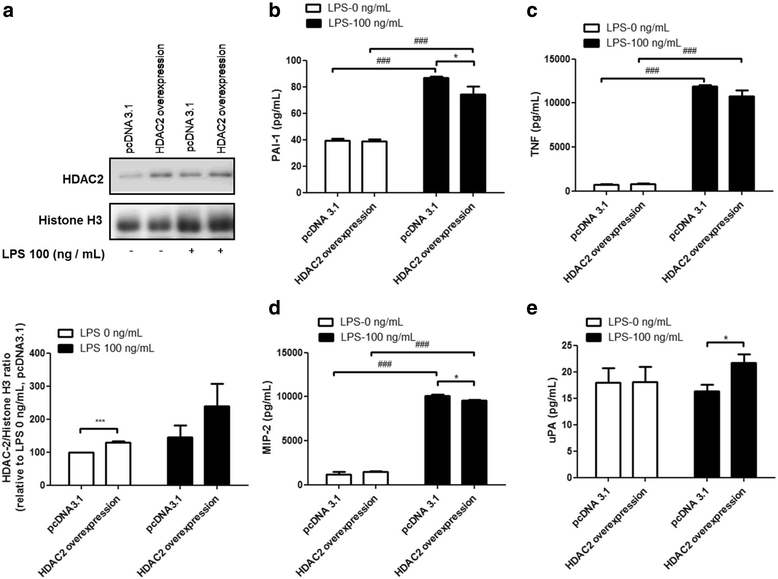
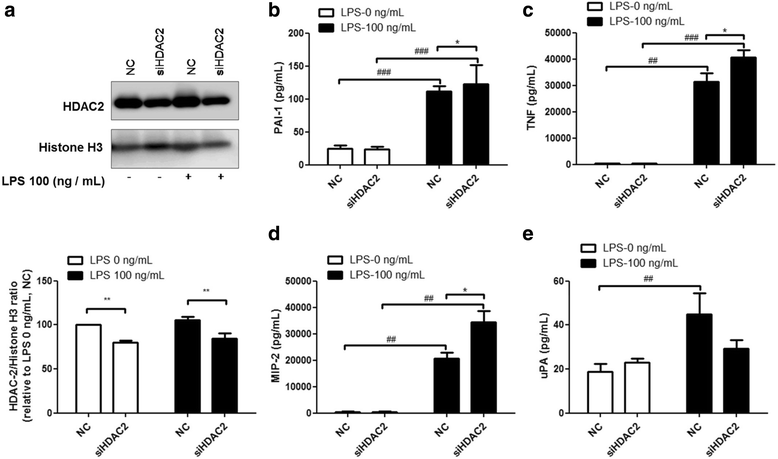
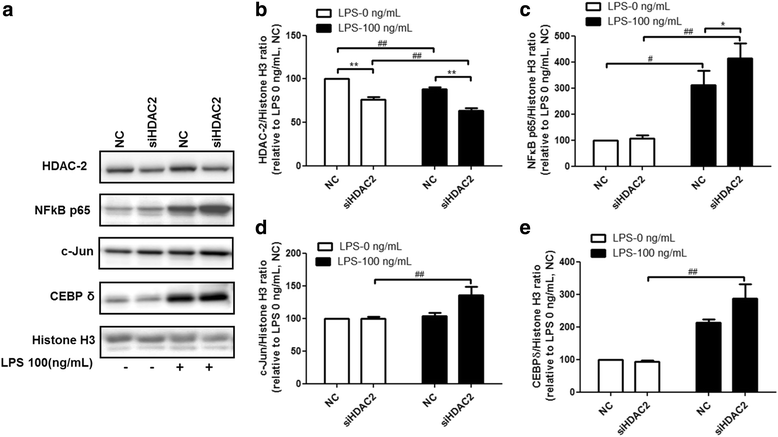
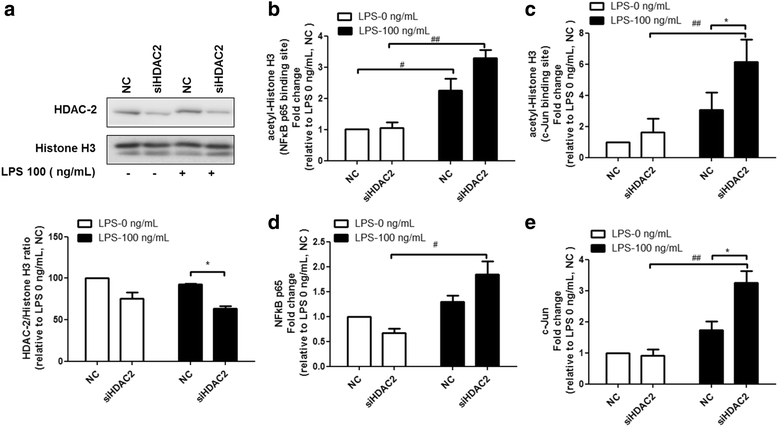
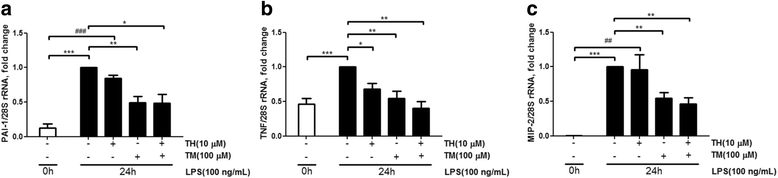
Results: Results revealed that PAI-1, TNF, and MIP-2 expression were inhibited by theophylline, an HDAC2 enhancer, in a RAW macrophage cell line, following LPS-induced inflammation. Thus, HDAC2 plays an important role in immune defense by regulating the expression of inflammatory genes via the c-Jun/PAI-1 pathway. During LPS-induced inflammation, overexpression of HDAC2 was found to inhibit PAI-1, TNF, and MIP-2 expression. Following LPS stimulation, HDAC2 knockdown increased nuclear translocation and DNA binding of c-Jun to the PAI-1 gene promoter, thereby activating PAI-1 gene transcription. Furthermore, inhibition of PAI-1 by TM5275 alone or in combination with theophylline notably suppressed TNF and MIP-2 expression.
Conclusion: HDAC2 can attenuate lipopolysaccharide-induced inflammation by regulating c-Jun and PAI-1 expression in macrophages.
Keywords: Histone deacetylase 2 (HDAC2); Lipopolysaccharide (LPS); Plasminogen activator inhibitor (PAI).
Conflict of interest statement
Procedures for the care of the animals and experiments were followed in accordance with the Guide of the Care and Use of Laboratory Animals from Chang Gung Memorial Hospital institutional animal care and use committee. The Institutional Biosafety Committee of Chang Gung University approved the experimental procedures.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. - DOI - PMC - PubMed
-
- Fang WF, Douglas IS, Wang CC, Kao HC, Chang YT, Tseng CC, Huang KT, Chang HC, Lin MC. 5-lipoxygenase activating protein (FLAP) dependent leukotriene biosynthesis inhibition (MK591) attenuates lipid a endotoxin-induced inflammation. PLoS One. 2014;9:e102622. doi: 10.1371/journal.pone.0102622. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

