A Case of Immune Thrombocytopenia as a Rare Side Effect of an Immunotherapy with PD1-Blocking Agents for Metastatic Melanoma
- PMID: 29344020
- PMCID: PMC5757577
- DOI: 10.1159/000479237
A Case of Immune Thrombocytopenia as a Rare Side Effect of an Immunotherapy with PD1-Blocking Agents for Metastatic Melanoma
Abstract
Background: Checkpoint blocking agents such as pembrolizumab or nivolumab may induce a diversity of mostly autoimmune-mediated side effects. These autoimmune phenomena mainly affect ductless glands such as the pituitary gland (hypophysitis), the thyroid gland (thyreoiditis), the skin (vitiligo and rash), the colon (colitis), and the lung (pneumonitis). Furthermore, many other organs or organ systems may be affected.
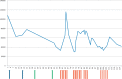
Case report: This work describes a case of an immune thrombocytopenia that developed or rather became clinically significant shortly after initiation of a systemic therapy with first nivolumab and later pembrolizumab given due to metastatic melanoma. Platelet counts before this systemic therapy were slightly decreased with values around 110/nl (normal value 140-400/nl). Thrombocytopenia developed or became apparent rapidly within 10 days after the first intravenous application of nivolumab and worsened after changeover to pembrolizumab. Therapy had to be stopped due to disease progression and steady aggravation of thrombocytopenia. Immune hematology assays could prove an autoimmune mediated genesis of thrombocytopenia.
Conclusion: Checkpoint inhibitors may induce a multiplicity of mostly autoimmune-mediated side effects. In contrast to chemotherapy-induced cytopenia that results from bone marrow toxicity, thrombocytopenia in melanoma patients treated with checkpoint inhibiting substances seems to result from autoimmune-mediated side effects in the majority of the cases. Thorough laboratory controls during these therapies are therefore required. In case of thrombocytopenia, immune hematology testing to diagnose or rule out immune thrombocytopenia is indispensable.
Keywords: Checkpoint blockade; Immune thrombocytopenia; Melanoma; Nivolumab; Pembrolizumab.
Figures
References
-
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. - PubMed
-
- Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, Patnaik A, Dronca R, Zarour H, Joseph RW, Boasberg P, Chmielowski B, Mateus C, Postow MA, Gergich K, Elassaiss-Schaap J, Li XN, Iannone R, Ebbinghaus SW, Kang SP, Daud A. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. - PubMed
-
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, investigators K- Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. - PubMed
-
- Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. - PubMed
-
- Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, Schmidgen MI, Gutzmer R, Utikal JS, Goppner D, Hassel JC, Meier F, Tietze JK, Thomas I, Weishaupt C, Leverkus M, Wahl R, Dietrich U, Garbe C, Kirchberger MC, Eigentler T, Berking C, Gesierich A, Krackhardt AM, Schadendorf D, Schuler G, Dummer R, Heinzerling LM. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources