Repository corticotropin injection in patients with persistently active SLE requiring corticosteroids: post hoc analysis of results from a two-part, 52-week pilot study
- PMID: 29344387
- PMCID: PMC5761300
- DOI: 10.1136/lupus-2017-000240
Repository corticotropin injection in patients with persistently active SLE requiring corticosteroids: post hoc analysis of results from a two-part, 52-week pilot study
Abstract
Objective: Post hoc analyses evaluated the effectiveness and safety of repository corticotropin injection (RCI) in patients with persistently active SLE over 52 weeks.
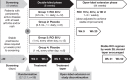
Methods: Patients were initially randomised to 40 U daily or 80 U every other day RCI (n=26) or placebo (n=12) for the 8-week double-blind period. Completers entered the open-label extension (OLE; n=33) receiving 16, 40 or 80 U RCI 1-3 times/week and were followed through week 52. Outcomes included proportion of responders based on a novel index (resolution of joint or skin activity using hybrid Systemic Lupus Erythematosus Disease Activity Index (hSLEDAI) without any worsening British Isles Lupus Assessment Group (BILAG) scores in other organ systems) or revised novel index (using SLE Responder Index (SRI) definition of BILAG worsening (1A or 2B)), proportion of responders by SRI and changes in total hSLEDAI and BILAG scores. Adverse events and laboratory values were assessed.
Results: At week 52, 12.0% (3/25) RCI/RCI patients and 36.4% (4/11) placebo/RCI patients were responders using the novel index. The revised novel responder index demonstrated response rates of 48.0% (12/25) and 54.5% (6/11) in the RCI/RCI and placebo/RCI groups, respectively. Proportions of SRI responders were 40.0% (10/25) and 54.5% (6/11). In the RCI/RCI group, total hSLEDAI and BILAG scores declined from 10.0 and 15.7 at week 0 to 3.5 and 4.6 at week 52, respectively. Reductions in the placebo/RCI group on switching were observed (mean hSLEDAI: 9.1-3.3; BILAG: 13.5-2.6). Other disease activity endpoints also improved in both groups. No new safety signals were observed during the OLE.
Conclusions: RCI demonstrated durable effectiveness in patients with persistently active SLE despite moderate-dose corticosteroid therapy. Switching from placebo resulted in reduced disease activity during the OLE. These data provide the foundation for evaluation of RCI in a robustly powered study.
Keywords: Clinical Trials; clinical research; corticotropin; disease activity; systemic lupus erythematosus.
Conflict of interest statement
Competing interests: RAF has been a paid consultant to Mallinckrodt Pharmaceuticals as well as the study and a site principal investigator. MM is a paid consultant to Mallinckrodt Pharmaceuticals. EZ is an employee of Mallinckrodt Pharmaceuticals. PMB is an employee of Mallinckrodt Pharmaceuticals and holds stock or stock options in the company.
Figures

References
-
- Gottschalk TA, Tsantikos E, Hibbs ML. Pathogenic inflammation and its therapeutic targeting in systemic lupus erythematosus. Front Immunol 2015;6:550 doi:10.3389/fimmu.2015.00550 - DOI - PMC - PubMed
-
- Bailey T, Rowley K, Bernknopf A. A review of systemic lupus erythematosus and current treatment options. Formulary 2011;46:178–94.
-
- Liao X, Pirapakaran T, Luo XM. Chemokines and chemokine receptors in the development of lupus nephritis. Mediators Inflamm 2016;2016:1–15. doi:10.1155/2016/6012715 - DOI - PMC - PubMed
-
- Arnaud L, Fagot JP, Mathian A, et al. Prevalence and incidence of systemic lupus erythematosus in France: a 2010 nation-wide population-based study. Autoimmun Rev 2014;13:1082–9. doi:10.1016/j.autrev.2014.08.034 - DOI - PubMed
-
- Gordon C, Amissah-Arthur MB, Gayed M, et al. British Society for Rheumatology Standards, Audit and Guidelines Working Group. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology 2018;57:e1–e45. doi:10.1093/rheumatology/kex286 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
