Prognostic significance of cytogenetic heterogeneity in patients with newly diagnosed multiple myeloma
- PMID: 29344579
- PMCID: PMC5761630
- DOI: 10.1182/bloodadvances.2017013334
Prognostic significance of cytogenetic heterogeneity in patients with newly diagnosed multiple myeloma
Abstract
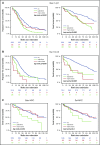
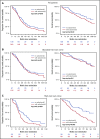
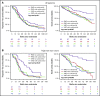
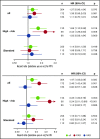
We investigated subclonal cytogenetic aberrations (CA) detected by interphase fluorescence in situ hybridization (iFISH) in patients with newly diagnosed multiple myeloma (MM) enrolled in the Haemato Oncology Foundation for Adults in the Netherlands (HOVON)-65/German-Speaking MM Group (GMMG)-HD4 phase 3 trial. Patients were either treated with 3 cycles of vincristine, Adriamycin, and dexamethasone or bortezomib, Adriamycin, and dexamethasone and then thalidomide or bortezomib maintenance after tandem autologous transplantation. Subclones were defined either by presence of different copy numbers of the same chromosome loci and/or CA present in at least 30% less and maximally 2/3 of cells compared with the main clone CA. Patients with subclones harbored more frequently high risk (31.0%) or hyperdiploid main clone aberrations (24.8%) than patients with t(11;14) in the main clone (10.1%). Gains and deletions of c-MYC were the only CA that occurred more frequently as subclone (8.1%/20.5%) than main clone (6.2%/3.9%, respectively). Treatment with bortezomib completely overcame the negative prognosis of high-risk CA in patients without subclones, but not in patients with additional subclonal CA. High-risk patients treated without bortezomib showed dismal outcome whether subclones were present or not. Cytogenetic heterogeneity defined by subclonal CA is of major prognostic significance in newly diagnosed MM patients treated with bortezomib within the HOVON-65/GMMG-HD4 trial.
Conflict of interest statement
Conflict-of-interest disclosure: M.M. received travel grants from Celgene, Abbvie, Amgen, and Janssen and acts on advisory boards for Amgen. D.H. received research funding from Sanofi and EngMab, as well as personal fees outside this work from Celgene. H.S. received speakers honoraria and travel grants from Celgene, Amgen, and Sanofi. I.G.H.S.-W. received research funding from Janssen and Novartis. C.S. received funding outside this work from Novartis, Celgene, and Janssen. K.C.W. received consultancy honoraria from Amgen, Takeda, and BMS, as well as honoraria from Novartis, research funding from Janssen and Celgene, and consultancy honoraria from Onyx. H.G. received consultancy honoraria and research funding from Janssen, Celgene, and Novartis, as well as consultancy honoraria from Onyx and Millenium and fees outside this work from BMS. J.H. received speakers honoraria from Celgene, acts on advisory boards for Janssen, BMS, and Amgen, and received consultancy honoraria from Janssen, Novartis, and Amgen as well as research funding from Sanofi. The remaining authors declare no competing financial interests.
Figures

References
-
- Bochtler T, Stölzel F, Heilig CE, et al. . Clonal heterogeneity as detected by metaphase karyotyping is an indicator of poor prognosis in acute myeloid leukemia. J Clin Oncol. 2013;31(31):3898-3905. - PubMed
-
- Manier S, Salem KZ, Park J, et al. . Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2016;14(2):100-113. - PubMed
-
- Moreau P, Masszi T, Grzasko N, et al. ; TOURMALINE-MM1 Study Group. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621-1634. - PubMed
-
- Leleu X, Karlin L, Macro M, et al. ; Intergroupe Francophone du Myélome (IFM). Pomalidomide plus low-dose dexamethasone in multiple myeloma with deletion 17p and/or translocation (4;14): IFM 2010-02 trial results. Blood. 2015;125(9):1411-1417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

