MGMT assessment in pituitary adenomas: comparison of different immunohistochemistry fixation chemicals
- PMID: 29344904
- PMCID: PMC5942339
- DOI: 10.1007/s11102-018-0862-x
MGMT assessment in pituitary adenomas: comparison of different immunohistochemistry fixation chemicals
Abstract
Purpose: Despite the established role of O6-methyl-guanine-DNA methyltransferase (MGMT) as a marker for temozolomide response, consensus of the most reliable method to assess MGMT expression in pituitary adenomas is still missing. Currently, immunohistochemistry (IHC) assessment of formaldehyde fixed tissue samples is most widely used in a semiquantitative description. As formaldehyde fails to completely preserve nucleic acids, RCL2, an alcohol-based formaldehyde-free fixative, has been proposed as a more reliable alternative in terms of cell stability. Furthermore, as the current method of IHC is semiquantitative and observer-dependent, pyrosequencing, an objective tool to evaluate the methylation status of the MGMT promoter, has emerged as a reliable and accurate alternative. The aim of this study was to validate the current IHC method for assessment of MGMT protein expression in pituitary adenomas.
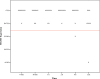
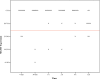
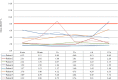
Methods: The tissue samples of 8 macroadenomas with positive IHC MGMT expression (> 50%) were investigated: first, we compared the time dependent stability of MGMT protein expression after pituitary adenoma removal between formaldehyde vs. RCL2. Then, we compared positive IHC MGMT expression with methylated promoter status using pyrosequencing.
Results: In the first 12 h after adenoma removal, tissue samples remained MGMT positive in significantly more samples when fixated with formaldehyde than with RCL2, respectively (96 vs. 81%, p = 0.025).
Conclusion: Our data confirm that the current method using formaldehyde tissue fixation and IHC reveals stable and reliable results of MGMT assessment in pituitary adenomas.
Keywords: MGMT; Pituitary adenoma; Promoter methylation; Time dependent.
Conflict of interest statement
All authors have no financial interest/arrangement or affiliation with one or more organizations that could be perceived as a real or apparent conflict of interest in the context of the article.
Figures

Similar articles
-
MGMT promoter methylation and immunoexpression in aggressive pituitary adenomas and carcinomas.J Neurooncol. 2011 Sep;104(3):647-57. doi: 10.1007/s11060-011-0532-6. Epub 2011 Feb 11. J Neurooncol. 2011. PMID: 21311951
-
Temozolomide treatment in aggressive pituitary tumors and pituitary carcinomas: a French multicenter experience.J Clin Endocrinol Metab. 2010 Oct;95(10):4592-9. doi: 10.1210/jc.2010-0644. Epub 2010 Jul 21. J Clin Endocrinol Metab. 2010. PMID: 20660056
-
Implications of MGMT methylation status in pituitary adenoma.Pathol Res Pract. 2014 Jul;210(7):407-11. doi: 10.1016/j.prp.2014.02.010. Epub 2014 Mar 12. Pathol Res Pract. 2014. PMID: 24690322
-
O6-methylguanine DNA methyltransferase gene promoter methylation status in gliomas and its correlation with other molecular alterations: first Indian report with review of challenges for use in customized treatment.Neurosurgery. 2010 Dec;67(6):1681-91. doi: 10.1227/NEU.0b013e3181f743f5. Neurosurgery. 2010. PMID: 21107199 Review.
-
Molecular status of pituitary carcinoma and atypical adenoma that contributes the effectiveness of temozolomide.Med Mol Morphol. 2014 Mar;47(1):1-7. doi: 10.1007/s00795-013-0050-z. Epub 2013 Aug 17. Med Mol Morphol. 2014. PMID: 23955641 Review.
Cited by
-
High-risk pituitary adenomas and strategies for predicting response to treatment.Hormones (Athens). 2022 Mar;21(1):1-14. doi: 10.1007/s42000-021-00333-y. Epub 2022 Jan 21. Hormones (Athens). 2022. PMID: 35061210 Review.
-
O6-Methylguanine-DNA Methyltransferase (MGMT): Challenges and New Opportunities in Glioma Chemotherapy.Front Oncol. 2020 Jan 17;9:1547. doi: 10.3389/fonc.2019.01547. eCollection 2019. Front Oncol. 2020. PMID: 32010632 Free PMC article. Review.
-
DNA Methylation in Pituitary Adenomas: A Scoping Review.Int J Mol Sci. 2025 Jan 10;26(2):531. doi: 10.3390/ijms26020531. Int J Mol Sci. 2025. PMID: 39859246 Free PMC article.
References
-
- Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates A, Dreno B, Henz M, Schadendorf D, Kapp A, Weiss J, Fraass U, Statkevich P, Muller M, Thatcher N. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–166. doi: 10.1200/JCO.2000.18.1.158. - DOI - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain., Radiotherapy T, G., National Cancer Institute of Canada Clinical Trials, G Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. - DOI - PubMed
-
- Bengtsson D, Schroder HD, Andersen M, Maiter D, Berinder K, Feldt Rasmussen U, Rasmussen AK, Johannsson G, Hoybye C, van der Lely AJ, Petersson M, Ragnarsson O, Burman P. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab. 2015;100(4):1689–1698. doi: 10.1210/jc.2014-4350. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

