Remarkable similarity in Plasmodium falciparum and Plasmodium vivax geranylgeranyl diphosphate synthase dynamics and its implication for antimalarial drug design
- PMID: 29345110
- PMCID: PMC6707526
- DOI: 10.1111/cbdd.13170
Remarkable similarity in Plasmodium falciparum and Plasmodium vivax geranylgeranyl diphosphate synthase dynamics and its implication for antimalarial drug design
Abstract
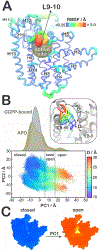
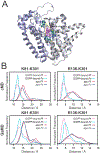
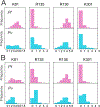
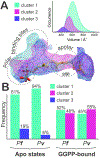
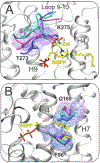
Malaria, mainly caused by Plasmodium falciparum and Plasmodium vivax, has been a growing cause of morbidity and mortality. P. falciparum is more lethal than is P. vivax, but there is a vital need for effective drugs against both species. Geranylgeranyl diphosphate synthase (GGPPS) is an enzyme involved in the biosynthesis of quinones and in protein prenylation and has been proposed to be a malaria drug target. However, the structure of P. falciparumGGPPS (PfGGPPS) has not been determined, due to difficulties in crystallization. Here, we created a PfGGPPS model using the homologous P.vivaxGGPPS X-ray structure as a template. We simulated the modeled PfGGPPS as well as PvGGPPS using conventional and Gaussian accelerated molecular dynamics in both apo- and GGPP-bound states. The MD simulations revealed a striking similarity in the dynamics of both enzymes with loop 9-10 controlling access to the active site. We also found that GGPP stabilizes PfGGPPS and PvGGPPS into closed conformations and via similar mechanisms. Shape-based analysis of the binding sites throughout the simulations suggests that the two enzymes will be readily targeted by the same inhibitors. Finally, we produced three MD-validated conformations of PfGGPPS to be used in future virtual screenings for potential new antimalarial drugs acting on both PvGGPPS and PfGGPPS.
Keywords: Plasmodium falciparum; Plasmodium vivax; GGPPS; Gaussian accelerated molecular dynamics simulations; Malaria; homology model; molecular dynamics simulations.
© 2018 John Wiley & Sons A/S.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures

Similar articles
-
Lipophilic analogs of zoledronate and risedronate inhibit Plasmodium geranylgeranyl diphosphate synthase (GGPPS) and exhibit potent antimalarial activity.Proc Natl Acad Sci U S A. 2012 Mar 13;109(11):4058-63. doi: 10.1073/pnas.1118215109. Epub 2012 Mar 5. Proc Natl Acad Sci U S A. 2012. PMID: 22392982 Free PMC article.
-
Dynamic Structure and Inhibition of a Malaria Drug Target: Geranylgeranyl Diphosphate Synthase.Biochemistry. 2016 Sep 13;55(36):5180-90. doi: 10.1021/acs.biochem.6b00398. Epub 2016 Sep 1. Biochemistry. 2016. PMID: 27564465 Free PMC article.
-
In Silico screening on the three-dimensional model of the Plasmodium vivax SUB1 protease leads to the validation of a novel anti-parasite compound.J Biol Chem. 2013 Jun 21;288(25):18561-73. doi: 10.1074/jbc.M113.456764. Epub 2013 May 7. J Biol Chem. 2013. PMID: 23653352 Free PMC article.
-
6-oxopurine phosphoribosyltransferase: a target for the development of antimalarial drugs.Curr Top Med Chem. 2011;11(16):2085-102. doi: 10.2174/156802611796575911. Curr Top Med Chem. 2011. PMID: 21619515 Review.
-
Current trends to design antimalarial drugs targeting N-myristoyltransferase.Future Microbiol. 2024;19(18):1601-1618. doi: 10.1080/17460913.2024.2412397. Epub 2024 Oct 23. Future Microbiol. 2024. PMID: 39440556 Review.
Cited by
-
Gaussian accelerated molecular dynamics (GaMD): principles and applications.Wiley Interdiscip Rev Comput Mol Sci. 2021 Sep-Oct;11(5):e1521. doi: 10.1002/wcms.1521. Epub 2021 Mar 1. Wiley Interdiscip Rev Comput Mol Sci. 2021. PMID: 34899998 Free PMC article.
-
MalDA, Accelerating Malaria Drug Discovery.Trends Parasitol. 2021 Jun;37(6):493-507. doi: 10.1016/j.pt.2021.01.009. Epub 2021 Feb 26. Trends Parasitol. 2021. PMID: 33648890 Free PMC article. Review.
-
Antimalarial drug discovery: progress and approaches.Nat Rev Drug Discov. 2023 Oct;22(10):807-826. doi: 10.1038/s41573-023-00772-9. Epub 2023 Aug 31. Nat Rev Drug Discov. 2023. PMID: 37652975 Free PMC article. Review.
-
Gaussian accelerated molecular dynamics for elucidation of drug pathways.Expert Opin Drug Discov. 2018 Nov;13(11):1055-1065. doi: 10.1080/17460441.2018.1538207. Epub 2018 Oct 29. Expert Opin Drug Discov. 2018. PMID: 30371112 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

