Mycobacterium tuberculosis: Rewiring host cell signaling to promote infection
- PMID: 29345343
- PMCID: PMC6446910
- DOI: 10.1002/JLB.4MR0717-277R
Mycobacterium tuberculosis: Rewiring host cell signaling to promote infection
Abstract
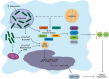
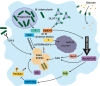
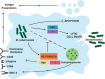
The ability of Mycobacterium tuberculosis to cause disease hinges upon successfully thwarting the innate defenses of the macrophage host cell. The pathogen's trump card is its armory of virulence factors that throw normal host cell signaling into disarray. This process of subverting the macrophage begins upon entry into the cell, when M. tuberculosis actively inhibits the fusion of the bacilli-laden phagosomes with lysosomes. The pathogen then modulates an array of host signal transduction pathways, which dampens the macrophage's host-protective cytokine response, while simultaneously adapting host cell metabolism to stimulate lipid body accumulation. Mycobacterium tuberculosis also renovates the surface of its innate host cells by altering the expression of key molecules required for full activation of the adaptive immune response. Finally, the pathogen coordinates its exit from the host cell by shifting the balance from the host-protective apoptotic cell death program toward a lytic form of host cell death. Thus, M. tuberculosis exploits its extensive repertoire of virulence factors in order to orchestrate the infection process to facilitate its growth, dissemination, and entry into latency. This review offers critical insights into the most recent advances in our knowledge of how M. tuberculosis manipulates host cell signaling. An appreciation of such interactions between the pathogen and host is critical for guiding novel therapies and understanding the factors that lead to the development of active disease in only a subset of exposed individuals.
Keywords: host-pathogen interactions; macrophages; virulence factors.
©2017 Society for Leukocyte Biology.
Figures
References
-
- Vynnycky E, Fine PEM. Lifetime risks, incubation period, and serial interval of tuberculosis. Am J Epidemiol. 2000;152:247–263. - PubMed
-
- Cambier CJ, Falkow S, Ramakrishnan L. Host evasion and exploitation schemes of Mycobacterium tuberculosis . Cell. 2014;159:1497–1509. - PubMed
-
- Sturgill‐Koszycki S, Schlesinger PH, Chakraborty P, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton‐ATPase. Science. 1994;263:678–681. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

