Mature gastric chief cells are not required for the development of metaplasia
- PMID: 29345968
- PMCID: PMC6732738
- DOI: 10.1152/ajpgi.00351.2017
Mature gastric chief cells are not required for the development of metaplasia
Abstract
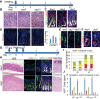
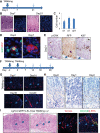
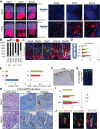
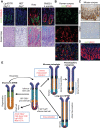
During human gastric carcinogenesis, intestinal metaplasia is frequently seen in the atrophic stomach. In mice, a distinct type of metaplasia known as spasmolytic polypeptide-expressing metaplasia (SPEM) is found in several inflammatory and genetically engineered models. Given the diversity of long- and short-term models of mouse SPEM, it remains unclear whether all models have a shared or distinct molecular mechanism. The origin of SPEM in mice is presently under debate. It is postulated that stem or progenitor cells acquire genetic alterations that then supply metaplastic cell clones, whereas the possibility of transdifferentiation or dedifferentiation from mature gastric chief cells has also been suggested. In this study, we report that loss of chief cells was sufficient to induce short-term regenerative SPEM-like lesions that originated from chief cell precursors in the gastric neck region. Furthermore, Lgr5+ mature chief cells failed to contribute to both short- and long-term metaplasia, whereas isthmus stem and progenitor cells efficiently contributed to long-term metaplasia. Interestingly, multiple administrations of high-dose pulsed tamoxifen induced expansion of Lgr5 expression and Lgr5-CreERT recombination within the isthmus progenitors apart from basal chief cells. Thus we conclude that short-term SPEM represents a regenerative process arising from neck progenitors following chief cell loss, whereas true long-term SPEM originates from isthmus progenitors. Mature gastric chief cells may be dispensable for SPEM development. NEW & NOTEWORTHY Recently, dedifferentiation ability in gastric chief cells during metaplasia development has been proposed. Our findings reveal that lesions that were thought to be acute metaplasia in fact represent normal regeneration supplied from neck lineage and that isthmus stem/progenitors are more responsible for sustained metaplastic changes. Cellular plasticity in gastric chief cells may be more limited than recently highlighted.
Keywords: Lgr5; gastric chief cell; metaplasia; stem cell.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
-
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36, 2010. doi: 10.1016/j.stem.2009.11.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

