Assessment of serum pharmacokinetics and urinary excretion of albendazole and its metabolites in human volunteers
- PMID: 29346367
- PMCID: PMC5773000
- DOI: 10.1371/journal.pntd.0005945
Assessment of serum pharmacokinetics and urinary excretion of albendazole and its metabolites in human volunteers
Abstract
Background: Soil Transmitted Helminth (STH) infections negatively impact physical and mental development in human populations. Current WHO guidelines recommend morbidity control of these infections through mass drug administration (MDA) using albendazole (ABZ) or mebendazole. Despite major reductions in STH associated morbidity globally, not all programs have demonstrated the expected impact on prevalence of parasite infections. These therapeutic failures may be related to poor programmatic coverage, suboptimal adherence or the exposure of parasites to sub-therapeutic drug concentrations. As part of the DeWorm3 project, we sought to characterize the serum disposition kinetics and pattern of urinary excretion of ABZ and its main metabolites ABZ sulphoxide (ABZSO) and ABZ sulphone (ABZSO2) in humans, and the assessment of the duration and optimal time point where ABZ and/or its metabolites can be measured in urine as an indirect assessment of an individual's adherence to treatment.
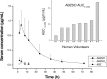
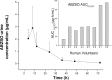
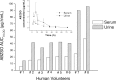
Methodology/principal findings: Consecutive venous blood and urine samples were collected from eight (8) human volunteers up to 72 h post-ABZ oral administration. ABZ/metabolites were quantified by HPLC. The ABZSO metabolite was the main analyte recovered both in serum and urine. ABZSO Cmax in serum was 1.20 ± 0.44 μg/mL, reached at 4.75 h post-treatment. In urine, ABZSO Cmax was 3.24 ± 1.51 μg/mL reached at 6.50 h post-ABZ administration.
Conclusion/significance: Pharmacokinetic data obtained for ABZ metabolites in serum and urine, including the recovery of the ABZ sulphoxide derivative up to 72 h in both matrixes and the recovery of the amino-ABZ sulphone metabolite in urine samples, are suggesting the possibility of developing a urine based method to assess compliance to ABZ treatment. Such an assay may be useful to optimize ABZ use in human patients.
Trial registration: ClinicalTrials.gov NCT03192449.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- WHO. First WHO report on neglected tropical diseases: working to overcome the global impact of neglected tropical diseases. World Heal Organ. 2010;1–184.
-
- Hotez PJ, Bundy D a P, Beegle K, Brooker S, Drake L, de Silva N, et al. Helminth Infections: Soil-transmitted Helminth Infections and Schistosomiasis. Dis Control Priorities Dev Ctries. 2006; 467–82.
-
- Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014; 7:37 doi: 10.1186/1756-3305-7-37 - DOI - PMC - PubMed
-
- Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P. Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin, and school performance. Cochrane database Syst Rev. 2015. doi: 10.1002/14651858.CD000371.pub6. - DOI - PMC - PubMed
-
- WHO. Assesing the efficacy of anthelminthic drugs against schistosomiasis and soil-transmitted helminthiasis World Heal Organ; 2013;1–29.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

