Oxytocin alters cell fate selection of rat neural progenitor cells in vitro
- PMID: 29346405
- PMCID: PMC5773179
- DOI: 10.1371/journal.pone.0191160
Oxytocin alters cell fate selection of rat neural progenitor cells in vitro
Abstract
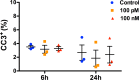
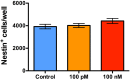
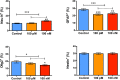
Synthetic oxytocin (sOT) is widely used during labor, yet little is known about its effects on fetal brain development despite evidence that it reaches the fetal circulation. Here, we tested the hypothesis that sOT would affect early neurodevelopment by investigating its effects on neural progenitor cells (NPC) from embryonic day 14 rat pups. NPCs expressed the oxytocin receptor (OXTR), which was downregulated by 45% upon prolonged treatment with sOT. Next, we examined the effects of sOT on NPC death, apoptosis, proliferation, and differentiation using antibodies to NeuN (neurons), Olig2 (oligodendrocytes), and GFAP (astrocytes). Treated NPCs were analysed with unbiased high-throughput immunocytochemistry. Neither 6 nor 24 h exposure to 100 pM or 100 nM sOT had an effect on viability as assessed by PI or CC-3 immunocytochemistry. Similarly, sOT had negligible effect on NPC proliferation, except that the overall rate of NPC proliferation was higher in the 24 h compared to the 6 h group regardless of sOT exposure. The most significant finding was that sOT exposure caused NPCs to select a predominantly neuronal lineage, along with a concomitant decrease in glial cells. Collectively, our data suggest that perinatal exposure to sOT can have neurodevelopmental consequences for the fetus, and support the need for in vivo anatomical and behavioral studies in offspring exposed to sOT in utero.
Conflict of interest statement
Figures







References
-
- Obstetrics ACoPB—. ACOG Practice Bulletin No. 107: Induction of labor. Obstetrics and gynecology. 2009;114(2 Pt 1):386–97. doi: 10.1097/AOG.0b013e3181b48ef5 . - DOI - PubMed
-
- Fuchs AR, Romero R, Keefe D, Parra M, Oyarzun E, Behnke E. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. American journal of obstetrics and gynecology. 1991;165(5 Pt 1):1515–23. . - PubMed
-
- Malek A, Blann E, Mattison DR. Human placental transport of oxytocin. The Journal of maternal-fetal medicine. 1996;5(5):245–55. doi: 10.1002/(SICI)1520-6661(199609/10)5:5<245::AID-MFM3>3.0.CO;2-H . - DOI - PubMed
-
- Yoshimura R, Kimura T, Watanabe D, Kiyama H. Differential expression of oxytocin receptor mRNA in the developing rat brain. Neuroscience research. 1996;24(3):291–304. . - PubMed
-
- Hammock EA, Levitt P. Oxytocin receptor ligand binding in embryonic tissue and postnatal brain development of the C57BL/6J mouse. Frontiers in behavioral neuroscience. 2013;7:195 doi: 10.3389/fnbeh.2013.00195 ; PubMed Central PMCID: PMC3858721. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

