The intracellular and intercellular cross-talk during subsidiary cell formation in Zea mays: existing and novel components orchestrating cell polarization and asymmetric division
- PMID: 29346521
- PMCID: PMC6215039
- DOI: 10.1093/aob/mcx193
The intracellular and intercellular cross-talk during subsidiary cell formation in Zea mays: existing and novel components orchestrating cell polarization and asymmetric division
Abstract
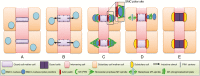
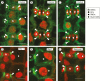
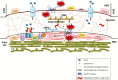
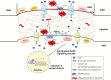
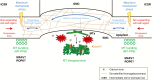
Background: Formation of stomatal complexes in Poaceae is the outcome of three asymmetric and one symmetric cell division occurring in particular leaf protodermal cells. In this definite sequence of cell division events, the generation of subsidiary cells is of particular importance and constitutes an attractive model for studying local intercellular stimulation. In brief, an induction stimulus emitted by the guard cell mother cells (GMCs) triggers a series of polarization events in their laterally adjacent protodermal cells. This signal determines the fate of the latter cells, forcing them to divide asymmetrically and become committed to subsidiary cell mother cells (SMCs).
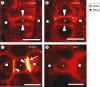
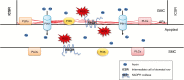
Scope: This article summarizes old and recent structural and molecular data mostly derived from Zea mays, focusing on the interplay between GMCs and SMCs, and on the unique polarization sequence occurring in both cell types. Recent evidence suggests that auxin operates as an inducer of SMC polarization/asymmetric division. The intercellular auxin transport is facilitated by the distribution of a specific transmembrane auxin carrier and requires reactive oxygen species (ROS). Interestingly, the local differentiation of the common cell wall between SMCs and GMCs is one of the earliest features of SMC polarization. Leucine-rich repeat receptor-like kinases, Rho-like plant GTPases as well as the SCAR/WAVE regulatory complex also participate in the perception of the morphogenetic stimulus and have been implicated in certain polarization events in SMCs. Moreover, the transduction of the auxin signal and its function are assisted by phosphatidylinositol-3-kinase and the products of the catalytic activity of phospholipases C and D.
Conclusion: In the present review, the possible role(s) of each of the components in SMC polarization and asymmetric division are discussed, and an overall perspective on the mechanisms beyond these phenomena is provided.
Figures


References
-
- Apostolakos P, Galatis B. 1987. Induction, polarity and spatial control of cytokinesis in some abnormal subsidiary cell mother cells of Zea mays. Protoplasma 140: 26–42.
-
- Apostolakos P, Panteris E, Galatis B. 2008. The involvement of phospholipases C and D in the asymmetric division of subsidiary cell mother cells of Zea mays. Cell Motility and the Cytoskeleton 65: 863–875. - PubMed
-
- Baluška F, Salaj J, Mathur J et al. . 2000. a Root hair formation: F-actin dependent tip growth is initiated by local assembly of profile-supported F-actin meshworks accumulated within expansin-enriched bulges. Developmental Biology 227: 618–632. - PubMed
-
- Baluška F, Volkmann D, Barlow PW. 2000b Actin-based domains of the ‘cell periphery complex’ and their associations with polarized ‘cell bodies’ in higher plants. Plant Biology 2: 253–267.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

