LC3 Immunostaining in the Inferior Olivary Nuclei of Cats With Niemann-Pick Disease Type C1 Is Associated With Patterned Purkinje Cell Loss
- PMID: 29346563
- PMCID: PMC5989620
- DOI: 10.1093/jnen/nlx119
LC3 Immunostaining in the Inferior Olivary Nuclei of Cats With Niemann-Pick Disease Type C1 Is Associated With Patterned Purkinje Cell Loss
Abstract
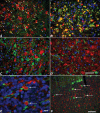
The feline model of Niemann-Pick disease, type C1 (NPC1) recapitulates the clinical, neuropathological, and biochemical abnormalities present in children with NPC1. The hallmarks of disease are the lysosomal storage of unesterified cholesterol and multiple sphingolipids in neurons, and the spatial and temporal distribution of Purkinje cell death. In feline NPC1 brain, microtubule-associated protein 1 light chain 3 (LC3) accumulations, indicating autophagosomes, were found within axons and presynaptic terminals. High densities of accumulated LC3 were seen in subdivisions of the inferior olive, which project to cerebellar regions that show the most Purkinje cell loss, suggesting that autophagic abnormalities in specific climbing fibers may contribute to the spatial pattern of Purkinje cell loss seen. Biweekly intrathecal administration of 2-hydroxypropyl-beta cyclodextrin (HPβCD) ameliorated neurological dysfunction, reduced cholesterol and sphingolipid accumulation, and increased lifespan in NPC1 cats. LC3 pathology was reduced in treated animals suggesting that HPβCD administration also ameliorates autophagic abnormalities. This study is the first to (i) identify specific brain regions exhibiting autophagic abnormalities in any species with NPC1, (ii) provide evidence of differential vulnerability among discrete brain nuclei and pathways, and (iii) show the amelioration of these abnormalities in NPC1 cats treated with HPβCD.
Keywords: Autophagy; Axonal degeneration; Cerebellar degeneration; LC3; Lysosomal storage disease; Neurodegeneration; Neuronal pathway.
© 2018 American Association of Neuropathologists, Inc. All rights reserved.
Figures










References
-
- Pentchev PG, Comly ME, Kruth HS, et al. A defect in cholesterol esterification in Niemann–Pick disease (type C) patients. Proc Natl Acad Sci USA 1985;82:8247–51http://dx.doi.org/10.1073/pnas.82.23.8247 - DOI - PMC - PubMed
-
- Vanier MT, Millat G.. Niemann–Pick disease type C. Clin Genet 2003;64:269–81http://dx.doi.org/10.1034/j.1399-0004.2003.00147.x - DOI - PubMed
-
- Runz H, Dolle D, Schlitter AM, et al. NPC-db, a Niemann–Pick type C disease gene variation database. Hum Mutat 2008;29:345–50http://dx.doi.org/10.1002/humu.20636 - DOI - PubMed
-
- Li H, Repa JJ, Valasek MA, et al. Molecular, anatomical, and biochemical events associated with neurodegeneration in mice with Niemann–Pick type C disease. J Neuropathol Exp Neurol 2005;64:323–33http://dx.doi.org/10.1093/jnen/64.4.323 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

