Receptor Usage of a Novel Bat Lineage C Betacoronavirus Reveals Evolution of Middle East Respiratory Syndrome-Related Coronavirus Spike Proteins for Human Dipeptidyl Peptidase 4 Binding
- PMID: 29346682
- PMCID: PMC7107427
- DOI: 10.1093/infdis/jiy018
Receptor Usage of a Novel Bat Lineage C Betacoronavirus Reveals Evolution of Middle East Respiratory Syndrome-Related Coronavirus Spike Proteins for Human Dipeptidyl Peptidase 4 Binding
Abstract
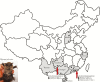
Although bats are known to harbor Middle East Respiratory Syndrome coronavirus (MERS-CoV)-related viruses, the role of bats in the evolutionary origin and pathway remains obscure. We identified a novel MERS-CoV-related betacoronavirus, Hp-BatCoV HKU25, from Chinese pipistrelle bats. Although it is closely related to MERS-CoV in most genome regions, its spike protein occupies a phylogenetic position between that of Ty-BatCoV HKU4 and Pi-BatCoV HKU5. Because Ty-BatCoV HKU4 but not Pi-BatCoV HKU5 can use the MERS-CoV receptor human dipeptidyl peptidase 4 (hDPP4) for cell entry, we tested the ability of Hp-BatCoV HKU25 to bind and use hDPP4. The HKU25-receptor binding domain (RBD) can bind to hDPP4 protein and hDPP4-expressing cells, but it does so with lower efficiency than that of MERS-RBD. Pseudovirus assays showed that HKU25-spike can use hDPP4 for entry to hDPP4-expressing cells, although with lower efficiency than that of MERS-spike and HKU4-spike. Our findings support a bat origin of MERS-CoV and suggest that bat CoV spike proteins may have evolved in a stepwise manner for binding to hDPP4.
Figures



References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367:1814–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

