The Apathy in Dementia Methylphenidate Trial 2 (ADMET 2): study protocol for a randomized controlled trial
- PMID: 29347996
- PMCID: PMC5774109
- DOI: 10.1186/s13063-017-2406-5
The Apathy in Dementia Methylphenidate Trial 2 (ADMET 2): study protocol for a randomized controlled trial
Abstract
Background: Alzheimer's disease (AD) is characterized not only by cognitive and functional decline, but also often by the presence of neuropsychiatric symptoms. Apathy, which can be defined as a lack of motivation, is one of the most prevalent neuropsychiatric symptoms in AD and typically leads to a worse quality of life and greater burden for caregivers. Treatment options for apathy in AD are limited, but studies have examined the use of the amphetamine, methylphenidate. The Apathy in Dementia Methylphenidate Trial (ADMET) found that treatment of apathy in AD with methylphenidate was associated with significant improvement in apathy in two of three outcome measures, some evidence of improvement in global cognition, and minimal adverse events. However, the trial only enrolled 60 participants who were followed for only 6 weeks. A larger, longer-lasting trial is required to confirm these promising findings.
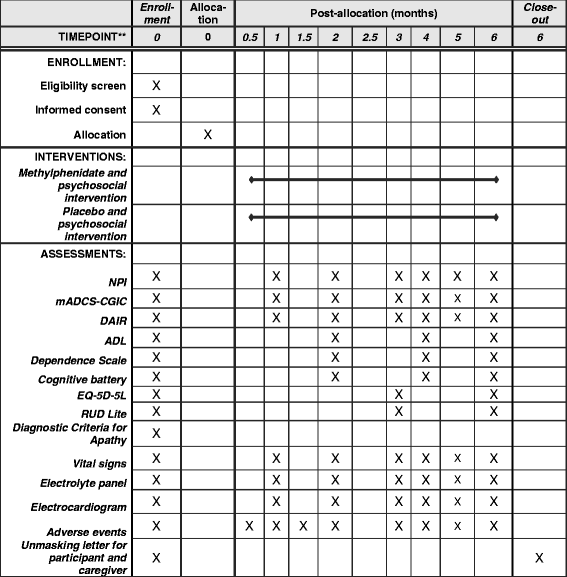
Methods: The Apathy in Dementia Methylphenidate Trial 2 (ADMET 2) is a phase III, placebo-controlled, masked, 6-month, multi-center, randomized clinical trial targeted to enroll 200 participants with AD and apathy. Participants are randomly assigned 1:1 to 20 mg methylphenidate per day prepared as four over-encapsulated tablets or to matching placebo. The primary outcomes include (1) the mean difference in the Neuropsychiatric Inventory Apathy subscale scores measured as change from baseline to 6 months, and (2) the odds of having a given rating or better on the modified AD Cooperative Study Clinical Global Impression of Change ratings at month 6 compared with the baseline rating. Other outcomes include change in cognition, safety, and cost-effectiveness measured at monthly follow-up visits up to 6 months.
Discussion: Given the prevalence of apathy in AD and its impact on both patients and caregivers, an intervention to alleviate apathy would be of great benefit to society. ADMET 2 follows on the promising results from the original ADMET to evaluate the efficacy of methylphenidate as a treatment for apathy in AD. With a larger sample size and longer follow up, ADMET 2 is poised to confirm or refute the original ADMET findings.
Trial registration: ClinicalTrials.gov, NCT02346201 . Registered on 26 January 2015.
Conflict of interest statement
Ethics approval and consent to participate
The institutional review board of the Johns Hopkins Bloomberg School of Public Health reviewed and approved the initial protocol on 9 July 2015, IRB nmber 00006303. Individual clinical centers received approval from their respective IRBs as follows: University of Arkansas, 7 April 2015, protocol 203507; Central Arkansas Veterans Healthcare System institutional review board, 17 August 2015, protocol 639677-3; Western Institutional Review Board, 16 August 2015, protocol 20150480; University Hospitals Case Medical Center institutional review board, 26 May 2015, protocol 05-15-11; Emory University institutional review board, 28 May 2015, IRB00080186; Johns Hopkins Medicine institutional review board, 6 April 2015; IRB00064958; University of Rochester institutional review board, 23 August 2015, RSRB 56395; Roper St. Francis institutional review board, 06 May 2015, protocol 736159-1; Sunnybrook Health Sciences Centre research ethics board, 24 September 2015, 505-2014; Wake Forest University Health Sciences institutional review board, 1 May 2015, IRB 31928; Yale university institutional review board, 10 June 2015, protocol 1503015481. These institutional review boards approved all subsequent amendments to the ADMET 2 protocol. ADMET 2 is being conducted in accordance with the Declaration of Helsinki. Informed consent from both the study participant and his or her caregiver is required prior to study entry and administration of any intervention. Copies of the informed consent template are attached as an appendix.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet (London, England). 2005;366(9503):2112-7. Epub 2005/12/20. https://doi.org/10.1016/s0140-6736(05)67889-0. PubMed PMID: 16360788; PubMed Central PMCID: PMCPMC2850264. - DOI - PMC - PubMed
-
- Rabins PV, Lyketsos CG, Steele CD. Pratical dementia care. 2. New York: Oxford University Press, Inc.; 2006.
-
- Benoit M, Berrut G, Doussaint J, Bakchine S, Bonin-Guillaume S, Fremont P, et al. Apathy and depression in mild Alzheimer’s disease: a cross-sectional study using diagnostic criteria. J Alzheimers Dis. 2012;31(2):325–34. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

