IFCC Working Group Recommendations for Assessing Commutability Part 2: Using the Difference in Bias between a Reference Material and Clinical Samples
- PMID: 29348165
- PMCID: PMC5835923
- DOI: 10.1373/clinchem.2017.277541
IFCC Working Group Recommendations for Assessing Commutability Part 2: Using the Difference in Bias between a Reference Material and Clinical Samples
Abstract
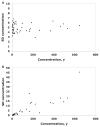
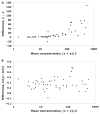
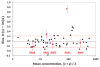
A process is described to assess the commutability of a reference material (RM) intended for use as a calibrator, trueness control, or external quality assessment sample based on the difference in bias between an RM and clinical samples (CSs) measured using 2 different measurement procedures (MPs). This difference in bias is compared with a criterion based on a medically relevant difference between an RM and CS results to make a conclusion regarding commutability. When more than 2 MPs are included, the commutability is assessed pairwise for all combinations of 2 MPs. This approach allows the same criterion to be used for all combinations of MPs included in the assessment. The assessment is based on an error model that allows estimation of various random and systematic sources of error, including those from sample-specific effects of interfering substances. An advantage of this approach is that the difference in bias between an RM and the average bias of CSs at the concentration (i.e., amount of substance present or quantity value) of the RM is determined and its uncertainty estimated. An RM is considered fit for purpose for those MPs for which commutability is demonstrated.
© 2017 American Association for Clinical Chemistry.
Conflict of interest statement
Figures

Comment in
-
The Enduring Importance and Challenge of Commutability.Clin Chem. 2018 Mar;64(3):421-423. doi: 10.1373/clinchem.2017.284216. Epub 2018 Jan 19. Clin Chem. 2018. PMID: 29352042 No abstract available.
References
-
- CLSI document EP14-A3. 3. Wayne (PA): Clinical and Laboratory Standards Institute; 2014. Evaluation of commutability of processed samples; approved guideline.
-
- CLSI document EP30-A. Wayne (PA): Clinical and Laboratory Standards Institute; 2010. Characterization and qualification of commutable reference materials for laboratory medicine; approved guideline.
-
- Nilsson G. Comparison of measurement methods based on a model for the error structure. J Chemometrics. 1991;5:523–36.
-
- Miller WG, Thienpont LM, Van Uytfanghe K, Clark PM, Lindstedt P, Nilsson G, Steffes MW. Toward standardization of insulin immunoassays. Clin Chem. 2009;55:1011–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

