Thiosulfate sulfurtransferase-like domain-containing 1 protein interacts with thioredoxin
- PMID: 29348167
- PMCID: PMC5827441
- DOI: 10.1074/jbc.RA117.000826
Thiosulfate sulfurtransferase-like domain-containing 1 protein interacts with thioredoxin
Abstract
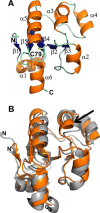
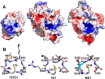
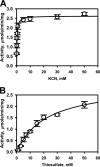
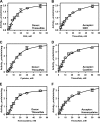
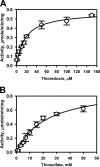
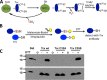
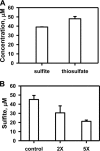
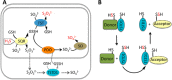
Rhodanese domains are structural modules present in the sulfurtransferase superfamily. These domains can exist as single units, in tandem repeats, or fused to domains with other activities. Despite their prevalence across species, the specific physiological roles of most sulfurtransferases are not known. Mammalian rhodanese and mercaptopyruvate sulfurtransferase are perhaps the best-studied members of this protein superfamily and are involved in hydrogen sulfide metabolism. The relatively unstudied human thiosulfate sulfurtransferase-like domain-containing 1 (TSTD1) protein, a single-domain cytoplasmic sulfurtransferase, was also postulated to play a role in the sulfide oxidation pathway using thiosulfate to form glutathione persulfide, for subsequent processing in the mitochondrial matrix. Prior kinetic analysis of TSTD1 was performed at pH 9.2, raising questions about relevance and the proposed model for TSTD1 function. In this study, we report a 1.04 Å resolution crystal structure of human TSTD1, which displays an exposed active site that is distinct from that of rhodanese and mercaptopyruvate sulfurtransferase. Kinetic studies with a combination of sulfur donors and acceptors reveal that TSTD1 exhibits a low Km for thioredoxin as a sulfane sulfur acceptor and that it utilizes thiosulfate inefficiently as a sulfur donor. The active site exposure and its interaction with thioredoxin suggest that TSTD1 might play a role in sulfide-based signaling. The apical localization of TSTD1 in human colonic crypts, which interfaces with sulfide-releasing microbes, and the overexpression of TSTD1 in colon cancer provide potentially intriguing clues as to its role in sulfide metabolism.
Keywords: crystal structure; enzyme kinetics; hydrogen sulfide; post-translational modification (PTM); sulfur; sulfur transferase.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Sodha N. R., Clements R. T., Feng J., Liu Y., Bianchi C., Horvath E. M., Szabo C., Stahl G. L., and Sellke F. W. (2009) Hydrogen sulfide therapy attenuates the inflammatory response in a porcine model of myocardial ischemia/reperfusion injury. J. Thorac. Cardiovasc. Surg. 138, 977–984 10.1016/j.jtcvs.2008.08.074 - DOI - PMC - PubMed
-
- Elrod J. W., Calvert J. W., Morrison J., Doeller J. E., Kraus D. W., Tao L., Jiao X., Scalia R., Kiss L., Szabo C., Kimura H., Chow C. W., and Lefer D. J. (2007) Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. U.S.A. 104, 15560–15565 10.1073/pnas.0705891104 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

