Multifunctional Role of Chymase in Acute and Chronic Tissue Injury and Remodeling
- PMID: 29348253
- PMCID: PMC5776735
- DOI: 10.1161/CIRCRESAHA.117.310978
Multifunctional Role of Chymase in Acute and Chronic Tissue Injury and Remodeling
Abstract
Chymase is the most efficient Ang II (angiotensin II)-forming enzyme in the human body and has been implicated in a wide variety of human diseases that also implicate its many other protease actions. Largely thought to be the product of mast cells, the identification of other cellular sources including cardiac fibroblasts and vascular endothelial cells demonstrates a more widely dispersed production and distribution system in various tissues. Furthermore, newly emerging evidence for its intracellular presence in cardiomyocytes and smooth muscle cells opens an entirely new compartment of chymase-mediated actions that were previously thought to be limited to the extracellular space. This review illustrates how these multiple chymase-mediated mechanisms of action can explain the residual risk in clinical trials of cardiovascular disease using conventional renin-angiotensin system blockade.
Keywords: angiotensin II; chymases; endothelial cells; mast cells; renin.
© 2018 American Heart Association, Inc.
Conflict of interest statement
Conflicts of Interest: None.
Figures

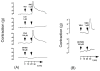

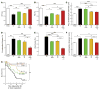

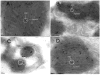
References
-
- Heutinck K, ten Berge I, Hack C, Hamann J, Rowshani A. Serine proteases of the human immune system in health and disease. Mol Immunol. 2010;47:1943–1955. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

