Macrophage sensing of single-walled carbon nanotubes via Toll-like receptors
- PMID: 29348435
- PMCID: PMC5773626
- DOI: 10.1038/s41598-018-19521-9
Macrophage sensing of single-walled carbon nanotubes via Toll-like receptors
Abstract
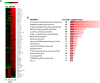
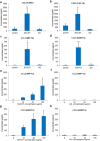
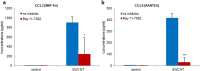
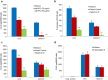
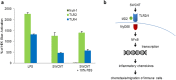
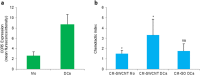
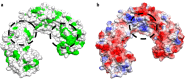
Carbon-based nanomaterials including carbon nanotubes (CNTs) have been shown to trigger inflammation. However, how these materials are 'sensed' by immune cells is not known. Here we compared the effects of two carbon-based nanomaterials, single-walled CNTs (SWCNTs) and graphene oxide (GO), on primary human monocyte-derived macrophages. Genome-wide transcriptomics assessment was performed at sub-cytotoxic doses. Pathway analysis of the microarray data revealed pronounced effects on chemokine-encoding genes in macrophages exposed to SWCNTs, but not in response to GO, and these results were validated by multiplex array-based cytokine and chemokine profiling. Conditioned medium from SWCNT-exposed cells acted as a chemoattractant for dendritic cells. Chemokine secretion was reduced upon inhibition of NF-κB, as predicted by upstream regulator analysis of the transcriptomics data, and Toll-like receptors (TLRs) and their adaptor molecule, MyD88 were shown to be important for CCL5 secretion. Moreover, a specific role for TLR2/4 was confirmed by using reporter cell lines. Computational studies to elucidate how SWCNTs may interact with TLR4 in the absence of a protein corona suggested that binding is guided mainly by hydrophobic interactions. Taken together, these results imply that CNTs may be 'sensed' as pathogens by immune cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Boraschi, D., Fadeel, B. & Duschl, A. Immune System. In: Adverse Effects of Engineered Nanomaterials: Exposure, Toxicology and Impact on Human Health (Second Edition). Eds. Fadeel, B., Pietroiusti, A., Shvedova, A. pp. 313–337. Elsevier (2017).
-
- Fadeel B. Clear and present danger? Engineered nanoparticles and the immune system. Swiss Med Wkly. 2012;142:w13609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

