Piperlongumine and p53-reactivator APR-246 selectively induce cell death in HNSCC by targeting GSTP1
- PMID: 29348462
- PMCID: PMC6014869
- DOI: 10.1038/s41388-017-0110-2
Piperlongumine and p53-reactivator APR-246 selectively induce cell death in HNSCC by targeting GSTP1
Abstract
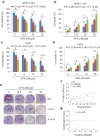
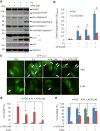
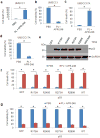
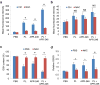
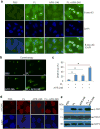
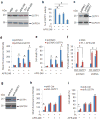
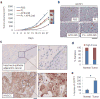
TP53 mutations frequently occur in head and neck squamous cell carcinoma (HNSCC) patients without human papillomavirus infection. The recurrence rate for these patients is distinctly high. It has been actively explored to identify agents that target TP53 mutations and restore wild-type (WT) TP53 activities in HNSCC. PRIMA-1 (p53-reactivation and induction of massive apoptosis-1) and its methylated analogue PRIMA-1Met (also called APR-246) were found to be able to reestablish the DNA-binding activity of p53 mutants and reinstate the functions of WT p53. Herein we report that piperlongumine (PL), an alkaloid isolated from Piper longum L., synergizes with APR-246 to selectively induce apoptosis and autophagic cell death in HNSCC cells, whereas primary and immortalized mouse embryonic fibroblasts and spontaneously immortalized non-tumorigenic human skin keratinocytes (HaCat) are spared from the damage by the co-treatment. Interestingly, PL-sensitized HNSCC cells to APR-246 are TP53 mutation-independent. Instead, we demonstrated that glutathione S-transferase pi 1 (GSTP1), a GST family member that catalyzes the conjugation of GSH with electrophilic compounds to fulfill its detoxification function, is highly expressed in HNSCC tissues. Administration of PL and APR-246 significantly suppresses GSTP1 activity, resulting in the accumulation of ROS, depletion of GSH, elevation of GSSG, and DNA damage. Ectopic expression of GSTP1 or pre-treatment with antioxidant N-acetyl-L-cysteine (NAC) abrogates the ROS elevation and decreases DNA damage, apoptosis, and autophagic cell death prompted by PL/APR-246. In addition, administration of PL and APR-246 impedes UMSCC10A xenograft tumor growth in SCID mice. Taken together, our data suggest that HNSCC cells are selectively sensitive to the combination of PL and APR-246 due to a remarkably synergistic effect of the co-treatment in the induction of ROS by suppression of GSTP1.
Conflict of interest statement
Figures
References
-
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

