Umbilical cord extracts improve osteoporotic abnormalities of bone marrow-derived mesenchymal stem cells and promote their therapeutic effects on ovariectomised rats
- PMID: 29348535
- PMCID: PMC5773568
- DOI: 10.1038/s41598-018-19516-6
Umbilical cord extracts improve osteoporotic abnormalities of bone marrow-derived mesenchymal stem cells and promote their therapeutic effects on ovariectomised rats
Erratum in
-
Author Correction: Umbilical cord extracts improve osteoporotic abnormalities of bone marrow-derived mesenchymal stem cells and promote their therapeutic effects on ovariectomised rats.Sci Rep. 2020 Dec 9;10(1):21987. doi: 10.1038/s41598-020-78836-8. Sci Rep. 2020. PMID: 33299025 Free PMC article. No abstract available.
Abstract
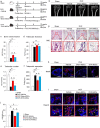
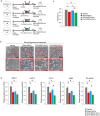
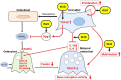
Bone marrow-derived mesenchymal stem cells (BM-MSCs) are the most valuable source of autologous cells for transplantation and tissue regeneration to treat osteoporosis. Although BM-MSCs are the primary cells responsible for maintaining bone metabolism and homeostasis, their regenerative ability may be attenuated in postmenopausal osteoporosis patients. Therefore, we first examined potential abnormalities of BM-MSCs in an oestrogen-deficient rat model constructed by ovariectomy (OVX-MSCs). Cell proliferation, mobilisation, and regulation of osteoclasts were downregulated in OVX-MSCs. Moreover, therapeutic effects of OVX-MSCs were decreased in OVX rats. Accordingly, we developed a new activator for BM-MSCs using human umbilical cord extracts, Wharton's jelly extract supernatant (WJS), which improved cell proliferation, mobilisation and suppressive effects on activated osteoclasts in OVX-MSCs. Bone volume, RANK and TRACP expression of osteoclasts, as well as proinflammatory cytokine expression in bone tissues, were ameliorated by OVX-MSCs activated with WJS (OVX-MSCs-WJ) in OVX rats. Fusion and bone resorption activity of osteoclasts were suppressed in macrophage-induced and primary mouse bone marrow cell-induced osteoclasts via suppression of osteoclast-specific genes, such as Nfatc1, Clcn7, Atp6i and Dc-stamp, by co-culture with OVX-MSCs-WJ in vitro. In this study, we developed a new activator, WJS, which improved the functional abnormalities and therapeutic effects of BM-MSCs on postmenopausal osteoporosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Umbilical cord extracts improve diabetic abnormalities in bone marrow-derived mesenchymal stem cells and increase their therapeutic effects on diabetic nephropathy.Sci Rep. 2017 Aug 16;7(1):8484. doi: 10.1038/s41598-017-08921-y. Sci Rep. 2017. PMID: 28814814 Free PMC article.
-
Xenograft of Human Umbilical Mesenchymal Stem Cells from Wharton's Jelly Differentiating into Osteocytes and Reducing Osteoclast Activity Reverses Osteoporosis in Ovariectomized Rats.Cell Transplant. 2018 Jan;27(1):194-208. doi: 10.1177/0963689717750666. Cell Transplant. 2018. PMID: 29562774 Free PMC article.
-
Differential expression of cell cycle and WNT pathway-related genes accounts for differences in the growth and differentiation potential of Wharton's jelly and bone marrow-derived mesenchymal stem cells.Stem Cell Res Ther. 2017 Apr 26;8(1):102. doi: 10.1186/s13287-017-0555-9. Stem Cell Res Ther. 2017. PMID: 28446235 Free PMC article.
-
Mesenchymal stem cells derived from Wharton's Jelly of the umbilical cord: biological properties and emerging clinical applications.Curr Stem Cell Res Ther. 2013 Mar;8(2):144-55. doi: 10.2174/1574888x11308020005. Curr Stem Cell Res Ther. 2013. PMID: 23279098 Review.
-
Mesenchymal Stem Cells from Wharton's Jelly and Amniotic Fluid.Best Pract Res Clin Obstet Gynaecol. 2016 Feb;31:30-44. doi: 10.1016/j.bpobgyn.2015.07.006. Epub 2015 Sep 10. Best Pract Res Clin Obstet Gynaecol. 2016. PMID: 26482184 Review.
Cited by
-
Use of Human Umbilical Cord and Its Byproducts in Tissue Regeneration.Front Bioeng Biotechnol. 2020 Mar 10;8:117. doi: 10.3389/fbioe.2020.00117. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32211387 Free PMC article.
-
Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis.Cell Prolif. 2021 Jan;54(1):e12956. doi: 10.1111/cpr.12956. Epub 2020 Nov 18. Cell Prolif. 2021. PMID: 33210341 Free PMC article. Review.
-
Mechanical force modulates periodontal ligament stem cell characteristics during bone remodelling via TRPV4.Cell Prolif. 2020 Oct;53(10):e12912. doi: 10.1111/cpr.12912. Epub 2020 Sep 22. Cell Prolif. 2020. PMID: 32964544 Free PMC article.
-
Bioinformatics analysis and identification of circular RNAs promoting the osteogenic differentiation of human bone marrow mesenchymal stem cells on titanium treated by surface mechanical attrition.PeerJ. 2020 Jul 13;8:e9292. doi: 10.7717/peerj.9292. eCollection 2020. PeerJ. 2020. PMID: 32742764 Free PMC article.
-
Impact of 3D cell culture on bone regeneration potential of mesenchymal stromal cells.Stem Cell Res Ther. 2021 Jan 7;12(1):31. doi: 10.1186/s13287-020-02094-8. Stem Cell Res Ther. 2021. PMID: 33413646 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

