Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling
- PMID: 29348540
- PMCID: PMC5833830
- DOI: 10.1038/s41419-017-0176-3
Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling
Abstract
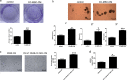
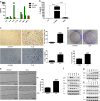
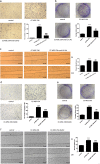
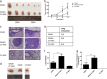
Mesenchymal stem cells (MSCs) have been reported to localize in colorectal carcinomas, and participate in the formation of the tumor microenvironment. They have recently been isolated from colorectal cancer tissues, and are implicated in the growth, invasion, and metastasis of cancer cells. However, the roles and detailed mechanisms associated with human colorectal cancer-derived MSCs (CC-MSCs) have not been fully addressed. In this study, we found that CC-MSCs increased the migration and invasion of colorectal cancer cells and promoted the tumorigenesis of colorectal cancer through epithelial-to-mesenchymal transition (EMT) in vitro. We also found that CC-MSCs enhanced the growth and metastasis of colorectal cancer in vivo. Mechanistically, we determined that interleukin-6 (IL-6) was the most highly expressed cytokine in the CC-MSC conditioned medium, and promoted the progression of colorectal cancer cells through IL-6/JAK2/STAT3 signaling, which activated PI3K/AKT signaling. We used anti-IL-6 antibody to target IL-6. Collectively, these results reveal that the IL-6 secreted by CC-MSCs enhances the progression of colorectal cancer cells through IL-6/JAK2/STAT3 signaling, and could provide a novel therapeutic or preventive target.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Mesenchymal stem cells promote formation of colorectal tumors in mice.Gastroenterology. 2011 Sep;141(3):1046-56. doi: 10.1053/j.gastro.2011.05.045. Epub 2011 May 31. Gastroenterology. 2011. PMID: 21699785
-
Acute myeloid leukemia (AML)-derived mesenchymal stem cells induce chemoresistance and epithelial-mesenchymal transition-like program in AML through IL-6/JAK2/STAT3 signaling.Cancer Sci. 2023 Aug;114(8):3287-3300. doi: 10.1111/cas.15855. Epub 2023 Jun 4. Cancer Sci. 2023. PMID: 37272257 Free PMC article.
-
[Gastric cancer-derived mesenchymal stem cells regulate the M2 polarization of macrophages within gastric cancer microenvironment via JAK2/STAT3 signaling pathway].Zhonghua Zhong Liu Za Zhi. 2022 Jul 23;44(7):728-736. doi: 10.3760/cma.j.cn112152-20200106-00008. Zhonghua Zhong Liu Za Zhi. 2022. PMID: 35880339 Chinese.
-
Potential functions and therapeutic implications of glioma-resident mesenchymal stem cells.Cell Biol Toxicol. 2023 Jun;39(3):853-866. doi: 10.1007/s10565-023-09808-7. Epub 2023 May 3. Cell Biol Toxicol. 2023. PMID: 37138122 Review.
-
Involvement of mesenchymal stem cells in cancer progression and metastases.Curr Cancer Drug Targets. 2015;15(2):88-98. doi: 10.2174/1568009615666150126154151. Curr Cancer Drug Targets. 2015. PMID: 25619387 Review.
Cited by
-
Targeting regulation of tryptophan metabolism for colorectal cancer therapy: a systematic review.RSC Adv. 2019 Jan 23;9(6):3072-3080. doi: 10.1039/c8ra08520j. eCollection 2019 Jan 22. RSC Adv. 2019. PMID: 35518968 Free PMC article. Review.
-
JAK2-Mediated Phosphorylation of Stress-Induced Phosphoprotein-1 (STIP1) in Human Cells.Int J Mol Sci. 2022 Feb 22;23(5):2420. doi: 10.3390/ijms23052420. Int J Mol Sci. 2022. PMID: 35269562 Free PMC article.
-
Human colorectal cancer derived-MSCs promote tumor cells escape from senescence via P53/P21 pathway.Clin Transl Oncol. 2020 Apr;22(4):503-511. doi: 10.1007/s12094-019-02152-5. Epub 2019 Jun 19. Clin Transl Oncol. 2020. PMID: 31218648
-
Reg3α and Reg3β Expressions Followed by JAK2/STAT3 Activation Play a Pivotal Role in the Acceleration of Liver Hypertrophy in a Rat ALPPS Model.Int J Mol Sci. 2020 Jun 7;21(11):4077. doi: 10.3390/ijms21114077. Int J Mol Sci. 2020. PMID: 32517345 Free PMC article.
-
Cancer Stem Cells and Its Role in Angiogenesis and Vasculogenic Mimicry in Gastrointestinal Cancers.Front Oncol. 2020 Mar 31;10:413. doi: 10.3389/fonc.2020.00413. eCollection 2020. Front Oncol. 2020. PMID: 32296643 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

