Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender
- PMID: 29348568
- PMCID: PMC5773506
- DOI: 10.1038/s41467-017-02648-0
Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender
Abstract
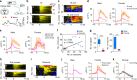
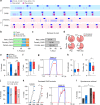
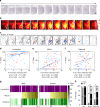
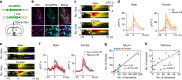
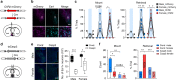
The medial preoptic area (mPOA) differs between males and females in nearly all species examined to date, including humans. Here, using fiber photometry recordings of Ca2+ transients in freely behaving mice, we show ramping activities in the mPOA that precede and correlate with sexually dimorphic display of male-typical mounting and female-typical pup retrieval. Strikingly, optogenetic stimulation of the mPOA elicits similar display of mounting and pup retrieval in both males and females. Furthermore, by means of recording, ablation, optogenetic activation, and inhibition, we show mPOA neurons expressing estrogen receptor alpha (Esr1) are essential for the sexually biased display of these behaviors. Together, these results underscore the shared layout of the brain that can mediate sex-specific behaviors in both male and female mice and provide an important functional frame to decode neural mechanisms governing sexually dimorphic behaviors in the future.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

