Adsorption and Desulfurization Mechanism of Thiophene on Layered FeS(001), (011), and (111) Surfaces: A Dispersion-Corrected Density Functional Theory Study
- PMID: 29348782
- PMCID: PMC5767879
- DOI: 10.1021/acs.jpcc.7b08711
Adsorption and Desulfurization Mechanism of Thiophene on Layered FeS(001), (011), and (111) Surfaces: A Dispersion-Corrected Density Functional Theory Study
Abstract
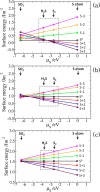
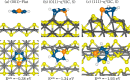
Layered transition-metal chalcogenides have emerged as a fascinating new class of materials for catalysis. Here, we present periodic density functional theory (DFT) calculations of the adsorption of thiophene and the direct desulfurization reaction pathways on the (001), (011), and (111) surfaces of layered FeS. The fundamental aspects of the thiophene adsorption, including the initial adsorption geometries, adsorption energies, structural parameters, and electronic properties, are presented. From the calculated adsorption energies, we show that the flat adsorption geometries, wherein the thiophene molecule forms multiple π-bonds with the FeS surfaces, are energetically more favorable than the upright adsorption geometries, with the strength of adsorption decreasing in the order FeS(111) > FeS(011) > FeS(001). The adsorption of the thiophene onto the reactive (011) and (111) surfaces is shown to be characterized by charge transfer from the interacting Fe d-band to the π-system of the thiophene molecule, which causes changes of the intramolecular structure including loss of aromaticity and elongation of the C-S bonds. The thermodynamic and kinetic analysis of the elementary steps involved in the direct desulfurization of thiophene on the reactive FeS surfaces is also presented. Direct desulfurization of thiophene occurs preferentially on the (111) surface, as reflected by the overall exothermic reaction energy calculated for the process (ER = -0.15 eV), with an activation energy of 1.58 eV.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Knudsen K. G.; Cooper B. H.; Topsøe H. Catalyst and process technologies for ultra-low sulfur diesel. Appl. Catal., A 1999, 189, 205–215. 10.1016/S0926-860X(99)00277-X. - DOI
-
- Song C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catal. Today 2003, 86, 211–263. 10.1016/S0920-5861(03)00412-7. - DOI
-
- Whitehurst D. D.; Isoda T.; Mochida I. Present State of the Art and Future Challenges in the Hydrodesulfurization of Polyaromatic Sulfur Compounds. Adv. Catal. 1998, 42, 345–471. 10.1016/S0360-0564(08)60631-8. - DOI
-
- Babich I. V.; Moulijn J. A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. 10.1016/S0016-2361(02)00324-1. - DOI
-
- Raybaud P.; Hafner J.; Kresse G.; Toulhoat H. Adsorption of thiophene on the catalytically active surface of MoS2: An ab initio local-density-functional study. Phys. Rev. Lett. 1998, 80, 1481–1484. 10.1103/PhysRevLett.80.1481. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
