Transfusion practice patterns in patients with anemia receiving myelosuppressive chemotherapy for nonmyeloid cancer: results from a prospective observational study
- PMID: 29349622
- PMCID: PMC5919983
- DOI: 10.1007/s00520-017-4035-7
Transfusion practice patterns in patients with anemia receiving myelosuppressive chemotherapy for nonmyeloid cancer: results from a prospective observational study
Abstract
Purpose: The decision to prescribe packed red blood cell (PRBC) transfusions in patients with chemotherapy-induced anemia (CIA) includes assessment of clinical features such as the patient's cancer type and treatment regimen, severity of anemia symptoms, and presence of comorbidities. We examined contemporary transfusion practices in patients with nonmyeloid cancer and CIA.
Methods: Key inclusion criteria were age ≥ 18 years with nonmyeloid cancer, receiving first/second-line myelosuppressive chemotherapy, baseline hemoglobin (Hb) ≤ 10.0 g/dL, and planned to receive ≥ 1 PRBC transfusions. Exclusion criteria were receipt of erythropoiesis-stimulating agents within 8 weeks of screening and/or chronic renal insufficiency. Data were collected from patients' medical records, laboratory values, and physician/provider questionnaires. Proportion of patients for each clinical consideration leading to a decision to prescribe a PRBC transfusion and 95% exact binomial confidence intervals were determined.
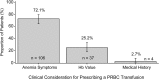
Results: The study enrolled 154 patients at 18 sites in USA; 147 (95.5%) received a PRBC transfusion. Fatigue was the most common symptom affecting the decision to prescribe a PRBC transfusion (101 [69.2%] patients). Of the three reasons selected as primary considerations for prescribing a PRBC transfusion, anemia symptoms (106 [72.1%] patients) was the most frequently reported, followed by Hb value (37 [25.2%] patients) and medical history (4 [2.7%] patients).
Conclusions: In this study, the primary consideration for prescribing a PRBC transfusion was anemia symptoms in 72.1% of patients, with only 25.2% of patients prescribed a transfusion based exclusively on Hb value. Results indicate that clinical judgment and patient symptoms, not just Hb value, were used in decisions to prescribe PRBC transfusions.
Keywords: Chemotherapy-induced anemia; Comorbidities; Fatigue; Hemoglobin; Packed red blood cell.
Conflict of interest statement
James Granfortuna, Kaye Shoffner, and Stephen E. DePasquale have no financial relationships or conflicts of interest to disclose. Sejal Badre was an employee of Amgen Inc. at the time of the study and owns/may have owned stock in Amgen Inc. Chet Bohac was an employee of Amgen Inc. at the time of the study and owns/may have owned stock in Amgen Inc.; he is currently an employee of and owns stock in Immune Design. Cisio De Oliveira Brandao is an employee of and owns stock in Amgen Inc.
Figures
References
-
- National Comprehensive Cancer Network. NCCN® clinical practice guidelines in oncology. Cancer- and chemotherapy-induced anemia version 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/anemia.pdf Accessed 8 December 2017
-
- Nieboer P, Buijs C, Rodenhuis S, Seynaeve C, Beex LV, van der Wall E, Richel DJ, Nooij MA, Voest EE, Hupperets P, Mulder NH, van der Graaf WT, TenVergert EM, van Tinteren H, de Vries EG. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296–8304. doi: 10.1200/JCO.2005.10.167. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

