Digital image analysis of Ki67 proliferation index in breast cancer using virtual dual staining on whole tissue sections: clinical validation and inter-platform agreement
- PMID: 29349710
- PMCID: PMC5882622
- DOI: 10.1007/s10549-018-4669-2
Digital image analysis of Ki67 proliferation index in breast cancer using virtual dual staining on whole tissue sections: clinical validation and inter-platform agreement
Abstract
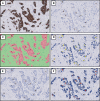
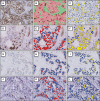
Purpose: The Ki67 proliferation index is a prognostic and predictive marker in breast cancer. Manual scoring is prone to inter- and intra-observer variability. The aims of this study were to clinically validate digital image analysis (DIA) of Ki67 using virtual dual staining (VDS) on whole tissue sections and to assess inter-platform agreement between two independent DIA platforms.
Methods: Serial whole tissue sections of 154 consecutive invasive breast carcinomas were stained for Ki67 and cytokeratin 8/18 with immunohistochemistry in a clinical setting. Ki67 proliferation index was determined using two independent DIA platforms, implementing VDS to identify tumor tissue. Manual Ki67 score was determined using a standardized manual counting protocol. Inter-observer agreement between manual and DIA scores and inter-platform agreement between both DIA platforms were determined and calculated using Spearman's correlation coefficients. Correlations and agreement were assessed with scatterplots and Bland-Altman plots.
Results: Spearman's correlation coefficients were 0.94 (p < 0.001) for inter-observer agreement between manual counting and platform A, 0.93 (p < 0.001) between manual counting and platform B, and 0.96 (p < 0.001) for inter-platform agreement. Scatterplots and Bland-Altman plots revealed no skewness within specific data ranges. In the few cases with ≥ 10% difference between manual counting and DIA, results by both platforms were similar.
Conclusions: DIA using VDS is an accurate method to determine the Ki67 proliferation index in breast cancer, as an alternative to manual scoring of whole sections in clinical practice. Inter-platform agreement between two different DIA platforms was excellent, suggesting vendor-independent clinical implementability.
Keywords: Breast cancer; Digital image analysis (DIA); Immunohistochemistry (IHC); Inter-platform agreement; Ki67 proliferation index; Virtual dual staining.
Conflict of interest statement
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical standards
All patient material was handled according to the ‘Code of conduct for health research’ of the Dutch Federation of Biomedical Scientific Societies [25]. Therefore, all experiments were performed in accordance to Dutch law and no additional permission from our Ethics Committee was required.
Figures

References
-
- American Cancer Society . Global cancer facts & figures. 3. Atlanta: American Cancer Society; 2015.
-
- Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumours of the breast. 4. Lyon: IARC Press; 2012.
-
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133(4):1710–1715. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

