Preclinical QSP Modeling in the Pharmaceutical Industry: An IQ Consortium Survey Examining the Current Landscape
- PMID: 29349875
- PMCID: PMC5869550
- DOI: 10.1002/psp4.12282
Preclinical QSP Modeling in the Pharmaceutical Industry: An IQ Consortium Survey Examining the Current Landscape
Abstract
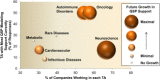
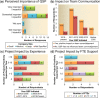
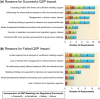
A cross-industry survey was conducted to assess the landscape of preclinical quantitative systems pharmacology (QSP) modeling within pharmaceutical companies. This article presents the survey results, which provide insights on the current state of preclinical QSP modeling in addition to future opportunities. Our results call attention to the need for an aligned definition and consistent terminology around QSP, yet highlight the broad applicability and benefits preclinical QSP modeling is currently delivering.
© 2018 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures

References
-
- DiMasi, J.A. , Grabowski, H.G. & Hansen, R.W. Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ. 47, 20–33 (2016). - PubMed
-
- Scannell, J.W. , Blanckley, A. , Boldon, H. & Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 11, 191–200 (2012). - PubMed
-
- Arrowsmith, J. & Miller, P. Trial watch: phase II and phase III attrition rates 2011–2012. Nat. Rev. Drug Discov. 12, 569 (2013). - PubMed
-
- Smietana, K. , Siatkowski, M. & Møller, M. Trends in clinical success rates. Nat. Rev. Drug Discov. 15, 379–380 (2016). - PubMed
-
- U.S. Department of Health and Human Services . Food and Drug Administration. Innovation or Stagnation: Challenge and opportunity on the critical path to new medical products. Washington, DC. <https://www.fda.gov/downloads/ScienceResearch/SpecialTopics/CriticalPath...> (2004).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

