Helenalin Analogues Targeting NF-κB p65: Thiol Reactivity and Cellular Potency Studies of Varied Electrophiles
- PMID: 29349898
- PMCID: PMC5894512
- DOI: 10.1002/cmdc.201700752
Helenalin Analogues Targeting NF-κB p65: Thiol Reactivity and Cellular Potency Studies of Varied Electrophiles
Abstract
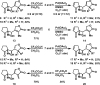
Helenalin is a pseudoguaianolide natural product that targets Cys38 within the DNA binding domain of NF-κB transcription factor p65 (RelA). Helenalin contains two Michael acceptors that covalently modify cysteines: a α-methylene-γ-butyrolactone and a cyclopentenone. We recently reported two simplified helenalin analogues that mimic the biological activity of helenalin and contain both electrophilic moieties. To determine the individual contributions of the Michael acceptors toward NF-κB inhibition, we synthesized a small library of helenalin-based analogues containing various combinations of α-methylene-γ-butyrolactones and cyclopentenones. The kinetics of thiol addition to a subset of the analogues was measured to determine the relative thiol reactivities of the embedded electrophiles. Additionally, the cellular NF-κB inhibitory activities of the analogues were determined to elucidate the contributions of each Michael acceptor to biological potency. Our studies suggest the α-methylene-γ-butyrolactone contributes most significantly to the NF-κB inhibition of our simplified helenalin analogues.
Keywords: Michael acceptors; bis-electrophiles; cysteine reactive; helenalin; p65/RelA transcription factor.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Xiao G, Fu J. Am. J. Cancer Res. 2011;1:192–221. - PMC - PubMed
- Aggarwal BB. Cancer Cell. 2004;6:203–208. - PubMed
- Herrington FD, Carmody RJ, Goodyear CS. J. Biomol. Screen. 2016;21:223–242. - PubMed
- Van der Heiden K, Cuhlmann S, Luong le A, Zakkar M, Evans PC. Clin. Sci. 2010;118:593–605. - PubMed
- Basseres DS, Baldwin AS. Oncogene. 2006;25:6817–6830. - PubMed
- Hayden MS, Ghosh S. Cell. 2008;132:344–362. - PubMed
- Huxford T, Ghosh G. Cold Spring Harbor Perspect. Biol. 2009;1:a000075. - PMC - PubMed
-
- Gilmore TD, Herscovitch M. Oncogene. 2006;25:6887–6899. - PubMed
-
- Lagoutte R, Patouret R, Winssinger N. Curr. Opin. Chem. Biol. 2017;39:54–63. - PubMed
- Huhn AJ, Guerra RM, Harvey EP, Bird GH, Walensky LD. Cell Chem. Biol. 2016;23:1123–1134. - PMC - PubMed
- Lagoutte R, Serba C, Abegg D, Hoch DG, Adibekian A, Winssinger N. Nat. Comm. 2016;7:12470. - PMC - PubMed
- Potashman MH, Duggan ME. J. Med. Chem. 2009;52:1231–1246. - PubMed
- Singh J, Petter RC, Baillie TA, Whitty A. Nat. Rev. Drug Discov. 2011;10:307–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

