Primary Liver Cancers-Part 1: Histopathology, Differential Diagnoses, and Risk Stratification
- PMID: 29350068
- PMCID: PMC5933592
- DOI: 10.1177/1073274817744625
Primary Liver Cancers-Part 1: Histopathology, Differential Diagnoses, and Risk Stratification
Abstract
Hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC) are the 2 most common primary malignant liver tumors, with hepatocellular and bile ductular differentiation, respectively. This article reviews the key histopathological findings of these 2 primary liver cancers and includes a review of the role of ancillary testing for differential diagnosis, risk stratification according to the American Joint Committee on Cancer (AJCC) staging recommendation, and a review of precancerous lesions. A literature review was conducted to identify articles with information relevant to precancerous precursors, current histopathological classification, ancillary testing, and risk stratification of primary malignant liver tumors. The histomorphology of normal liver, preinvasive precursors, primary malignancies, and morphological variants, and the utilization of ancillary tests for the pathological diagnosis are described. Dysplastic nodules are the preinvasive precursors of HCC, and intraductal papillary neoplasms of bile ducts and biliary intraepithelial neoplasia are the preinvasive precursors of CC. Benign liver nodules including focal nodular hyperplasia and adenomas are included in this review, since some forms of adenomas progress to HCC and often they have to be differentiated from well-differentiated HCC. A number of morphological variants of HCC have been described in the literature, and it is necessary to be aware of them in order to render the correct diagnosis. Risk stratification is still dependent on the AJCC staging system. The diagnosis of primary liver carcinomas is usually straightforward. Application of the appropriate ancillary studies aids in the differential diagnosis of difficult cases. The understanding of the carcinogenesis of these malignancies has improved with the standardization of the pathological classification of preinvasive precursors and studies of the molecular pathogenesis. Risk stratification still depends on pathological staging.
Keywords: biliary intraepithelial neoplasia; cholangiocarcinoma; dysplastic nodule; focal nodular hyperplasia; hepatic adenoma; hepatocellular carcinoma; intraductal papillary neoplasm.
Conflict of interest statement
Figures
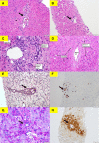

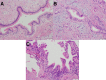

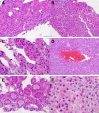

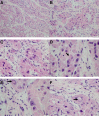
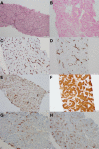
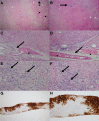

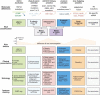
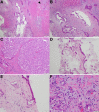

Similar articles
-
[Hepatocellular carcinoma and cholangiocarcinoma--different prognosis, pathogenesis and therapy].Zentralbl Chir. 2007 Aug;132(4):300-5. doi: 10.1055/s-2007-981195. Zentralbl Chir. 2007. PMID: 17724632 Review. German.
-
Preneoplastic lesions in the liver: Molecular insights and relevance for clinical practice.Liver Int. 2022 Mar;42(3):492-506. doi: 10.1111/liv.15152. Epub 2022 Jan 17. Liver Int. 2022. PMID: 34982503 Review.
-
Primary Liver Cancers, Part 2: Progression Pathways and Carcinogenesis.Cancer Control. 2018 Jan-Mar;25(1):1073274817744658. doi: 10.1177/1073274817744658. Cancer Control. 2018. PMID: 29353494 Free PMC article. Review.
-
[Selective deposition of agrin in the microvasculature of hepatocellular carcinoma: aspects in pathogenesis and differential diagnosis].Magy Onkol. 2008 Dec;52(4):379-83. doi: 10.1556/MOnkol.52.2008.4.7. Magy Onkol. 2008. PMID: 19068466 Hungarian.
-
The alphavbeta6 integrin is a highly specific immunohistochemical marker for cholangiocarcinoma.J Hepatol. 2010 Mar;52(3):362-9. doi: 10.1016/j.jhep.2009.12.006. Epub 2010 Jan 13. J Hepatol. 2010. PMID: 20137822
Cited by
-
MRI features of histologic subtypes of hepatocellular carcinoma: correlation with histologic, genetic, and molecular biologic classification.Eur Radiol. 2022 Aug;32(8):5119-5133. doi: 10.1007/s00330-022-08643-4. Epub 2022 Mar 8. Eur Radiol. 2022. PMID: 35258675 Review.
-
Therapy of Primary Liver Cancer.Innovation (Camb). 2020 Aug 28;1(2):100032. doi: 10.1016/j.xinn.2020.100032. Epub 2020 Aug 10. Innovation (Camb). 2020. PMID: 32914142 Free PMC article. Review.
-
Genomic Designs of rAAVs Contribute to Pathological Changes in the Livers and Spleens of Mice.Adv Cell Gene Ther. 2022;2022:6807904. doi: 10.1155/2022/6807904. Epub 2022 Mar 20. Adv Cell Gene Ther. 2022. PMID: 36507314 Free PMC article.
-
Mitogen-Activated Protein Kinases (MAPKs) and Cholangiocarcinoma: The Missing Link.Cells. 2019 Sep 28;8(10):1172. doi: 10.3390/cells8101172. Cells. 2019. PMID: 31569444 Free PMC article. Review.
-
Oncolytic Adenoviruses in Gastrointestinal Cancers.Biomedicines. 2018 Mar 11;6(1):33. doi: 10.3390/biomedicines6010033. Biomedicines. 2018. PMID: 29534501 Free PMC article. Review.
References
-
- International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995;22(3):983–993. - PubMed
-
- Watanabe S, Okita K, Harada T, et al. Morphologic studies of the liver cell dysplasia. Cancer. 1983;51(12):2197–2205. - PubMed
-
- Adachi E, Hashimoto H, Tsuneyoshi M. Proliferating cell nuclear antigen in hepatocellular carcinoma and small cell liver dysplasia. Cancer. 1993;72(10):2902–2909. - PubMed
-
- Plentz RR, Park YN, Lechel A, et al. Telomere shortening and inactivation of cell cycle checkpoints characterize human hepatocarcinogenesis. Hepatology. 2007;45(4):968–976. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

