Contribution of Mössbauer spectroscopy to the investigation of Fe/S biogenesis
- PMID: 29350298
- PMCID: PMC6006220
- DOI: 10.1007/s00775-018-1534-z
Contribution of Mössbauer spectroscopy to the investigation of Fe/S biogenesis
Erratum in
-
Correction to: Contribution of Mössbauer spectroscopy to the investigation of Fe/S biogenesis.J Biol Inorg Chem. 2018 Jun;23(4):645. doi: 10.1007/s00775-018-1572-6. J Biol Inorg Chem. 2018. PMID: 29860636 Free PMC article.
Abstract
Fe/S cluster biogenesis involves a complex machinery comprising several mitochondrial and cytosolic proteins. Fe/S cluster biosynthesis is closely intertwined with iron trafficking in the cell. Defects in Fe/S cluster elaboration result in severe diseases such as Friedreich ataxia. Deciphering this machinery is a challenge for the scientific community. Because iron is a key player, 57Fe-Mössbauer spectroscopy is especially appropriate for the characterization of Fe species and monitoring the iron distribution. This minireview intends to illustrate how Mössbauer spectroscopy contributes to unravel steps in Fe/S cluster biogenesis. Studies were performed on isolated proteins that may be present in multiple protein complexes. Since a few decades, Mössbauer spectroscopy was also performed on whole cells or on isolated compartments such as mitochondria and vacuoles, affording an overview of the iron trafficking. This minireview aims at presenting selected applications of 57Fe-Mössbauer spectroscopy to Fe/S cluster biogenesis.
Keywords: Fe/S biogenesis; Iron trafficking; Iron–sulfur cluster; Mössbauer spectroscopy.
Figures
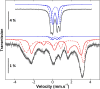
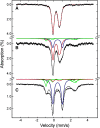
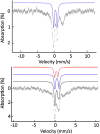
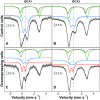
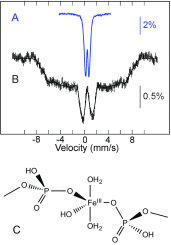
References
-
- Bill E. Iron–sulfur clusters—new features in enzymes and synthetic models. Hyperfine Interact. 2012;205:139–147. doi: 10.1007/s10751-011-0411-8. - DOI
-
- Münck E, Stubna A. 2.21—Mössbauer spectroscopy: bioinorganic A2—McCleverty, Jon A. In: Meyer TJ, editor. Comprehensive coordination chemistry II. Oxford: Pergamon; 2003. pp. 279–286.
-
- Gütlich P, Bill E, Trautwein AX. Mössbauer spectroscopy and transition metal chemistry: fundamentals and applications. Berlin Heidelberg: Springer; 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

