Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo
- PMID: 29351202
- PMCID: PMC5871973
- DOI: 10.3390/biom8010004
Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo
Abstract
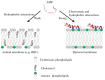
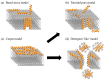
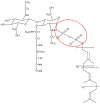
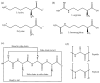
Antibiotic resistance is projected as one of the greatest threats to human health in the future and hence alternatives are being explored to combat resistance. Antimicrobial peptides (AMPs) have shown great promise, because use of AMPs leads bacteria to develop no or low resistance. In this review, we discuss the diversity, history and the various mechanisms of action of AMPs. Although many AMPs have reached clinical trials, to date not many have been approved by the US Food and Drug Administration (FDA) due to issues with toxicity, protease cleavage and short half-life. Some of the recent strategies developed to improve the activity and biocompatibility of AMPs, such as chemical modifications and the use of delivery systems, are also reviewed in this article.
Keywords: antimicrobial peptides; biocompatibility; bioconjugation; chemical modification; delivery systems; mechanism of action; proteolysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Chatterjee S., Chatterjee S., Lad S.J., Phansalkar M.S., Rupp R.H., Ganguli B.N., Fehlhaber H.W., Kogler H. Mersacidin, a new antibiotic from Bacillus. Fermentation, isolation, purification and chemical characterization. J. Antibiot. (Tokyo) 1992;45:832–838. doi: 10.7164/antibiotics.45.832. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

