Telomeres: Implications for Cancer Development
- PMID: 29351238
- PMCID: PMC5796239
- DOI: 10.3390/ijms19010294
Telomeres: Implications for Cancer Development
Abstract
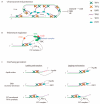
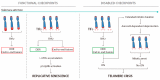
Telomeres facilitate the protection of natural ends of chromosomes from constitutive exposure to the DNA damage response (DDR). This is most likely achieved by a lariat structure that hides the linear telomeric DNA through protein-protein and protein-DNA interactions. The telomere shortening associated with DNA replication in the absence of a compensatory mechanism culminates in unmasked telomeres. Then, the subsequent activation of the DDR will define the fate of cells according to the functionality of cell cycle checkpoints. Dysfunctional telomeres can suppress cancer development by engaging replicative senescence or apoptotic pathways, but they can also promote tumour initiation. Studies in telomere dynamics and karyotype analysis underpin telomere crisis as a key event driving genomic instability. Significant attainment of telomerase or alternative lengthening of telomeres (ALT)-pathway to maintain telomere length may be permissive and required for clonal evolution of genomically-unstable cells during progression to malignancy. We summarise current knowledge of the role of telomeres in the maintenance of chromosomal stability and carcinogenesis.
Keywords: cancer; chromosome instability; telomere-dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Müller H.J. The remaking of chromosomes. Collect. Net-Woods Hole. 1938;13:181–198.
-
- Moyzis R.K., Buckingham J.M., Cram L.S., Dani M., Deaven L.L., Jones M.D., Meyne J., Ratliff R.L., Wu J.-R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

