Doxycycline modulates VEGF-A expression: Failure of doxycycline-inducible lentivirus shRNA vector to knockdown VEGF-A expression in transgenic mice
- PMID: 29351307
- PMCID: PMC5774698
- DOI: 10.1371/journal.pone.0190981
Doxycycline modulates VEGF-A expression: Failure of doxycycline-inducible lentivirus shRNA vector to knockdown VEGF-A expression in transgenic mice
Abstract
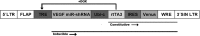
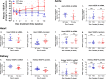
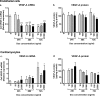
Vascular endothelial growth factor-A (VEGF-A) is the master regulator of angiogenesis, vascular permeability and growth. However, its role in mature blood vessels is still not well understood. To better understand the role of VEGF-A in the adult vasculature, we generated a VEGF-A knockdown mouse model carrying a doxycycline (dox)-regulatable short hairpin RNA (shRNA) transgene, which silences VEGF-A. The aim was to find the critical level of VEGF-A reduction for vascular well-being in vivo. In vitro, the dox-inducible lentiviral shRNA vector decreased VEGF-A expression efficiently and dose-dependently in mouse endothelial cells and cardiomyocytes. In the generated transgenic mice plasma VEGF-A levels decreased shortly after the dox treatment but returned back to normal after two weeks. VEGF-A expression decreased shortly after the dox treatment only in some tissues. Surprisingly, increasing the dox exposure time and dose led to elevated VEGF-A expression in some tissues of both wildtype and knockdown mice, suggesting that dox itself has an effect on VEGF-A expression. When the effect of dox on VEGF-A levels was further tested in naïve/non-transduced cells, the dox administration led to a decreased VEGF-A expression in endothelial cells but to an increased expression in cardiomyocytes. In conclusion, the VEGF-A knockdown was achieved in a dox-regulatable fashion with a VEGF-A shRNA vector in vitro, but not in the knockdown mouse model in vivo. Dox itself was found to regulate VEGF-A expression explaining the unexpected results in mice. The effect of dox on VEGF-A levels might at least partly explain its previously reported beneficial effects on myocardial and brain ischemia. Also, this effect on VEGF-A should be taken into account in all studies using dox-regulated vectors.
Conflict of interest statement
Figures


Similar articles
-
Lentiviral vector-mediated doxycycline-inducible USP39 shRNA or cDNA expression in triple-negative breast cancer cells.Oncol Rep. 2015 May;33(5):2477-83. doi: 10.3892/or.2015.3872. Epub 2015 Mar 20. Oncol Rep. 2015. PMID: 25812575
-
Inducible and reversible lentiviral and Recombination Mediated Cassette Exchange (RMCE) systems for controlling gene expression.PLoS One. 2015 Mar 13;10(3):e0116373. doi: 10.1371/journal.pone.0116373. eCollection 2015. PLoS One. 2015. PMID: 25768837 Free PMC article.
-
Doxycycline-dependent inducible and reversible RNA interference mediated by a single lentivirus vector.Biosci Biotechnol Biochem. 2013;77(4):776-81. doi: 10.1271/bbb.120917. Epub 2013 Apr 7. Biosci Biotechnol Biochem. 2013. PMID: 23563548
-
Tight control of transgene expression by lentivirus vectors containing second-generation tetracycline-responsive promoters.J Gene Med. 2005 Jun;7(6):803-17. doi: 10.1002/jgm.712. J Gene Med. 2005. PMID: 15655804
-
A novel lentivirus for quantitative assessment of gene knockdown in stem cell differentiation.Gene Ther. 2012 Dec;19(12):1123-32. doi: 10.1038/gt.2011.208. Epub 2012 Jan 12. Gene Ther. 2012. PMID: 22241174 Review.
Cited by
-
Toward Tightly Tuned Gene Expression Following Lentiviral Vector Transduction.Viruses. 2020 Dec 11;12(12):1427. doi: 10.3390/v12121427. Viruses. 2020. PMID: 33322556 Free PMC article. Review.
-
Effects of silencing epididymal vascular endothelial growth factor (VEGF) expression on hyaluronidase (HYD) activity in arsenic poisoning rats through downregulating VEGF receptor 2 (VEGFR2).Bioengineered. 2021 Dec;12(1):1351-1359. doi: 10.1080/21655979.2021.1915726. Bioengineered. 2021. PMID: 33904385 Free PMC article.
-
Correcting Calcium Dysregulation in Chronic Heart Failure Using SERCA2a Gene Therapy.Int J Mol Sci. 2018 Apr 5;19(4):1086. doi: 10.3390/ijms19041086. Int J Mol Sci. 2018. PMID: 29621141 Free PMC article. Review.
-
Direct Reprograming to Regenerate Myocardium and Repair Its Pacemaker and Conduction System.Medicines (Basel). 2018 Jun 4;5(2):48. doi: 10.3390/medicines5020048. Medicines (Basel). 2018. PMID: 29867004 Free PMC article. Review.
-
Glycogen synthase kinase-3β inhibits tubular regeneration in acute kidney injury by a FoxM1-dependent mechanism.FASEB J. 2020 Oct;34(10):13597-13608. doi: 10.1096/fj.202000526RR. Epub 2020 Aug 19. FASEB J. 2020. PMID: 32813289 Free PMC article.
References
-
- Roy H, Bhardwaj S, Yla-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett 2006. May 22;580(12):2879–2887. doi: 10.1016/j.febslet.2006.03.087 - DOI - PubMed
-
- Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011. December;2(12):1097–1105. doi: 10.1177/1947601911423031 - DOI - PMC - PubMed
-
- Smith GA, Fearnley GW, Harrison MA, Tomlinson DC, Wheatcroft SB, Ponnambalam S. Vascular endothelial growth factors: multitasking functionality in metabolism, health and disease. J Inherit Metab Dis 2015. April 14. - PubMed
-
- Yla-Herttuala S, Bridges C, Katz MG, Korpisalo P. Angiogenic gene therapy in cardiovascular diseases: dream or vision? Eur Heart J 2017. May 7;38(18):1365–1371. doi: 10.1093/eurheartj/ehw547 - DOI - PMC - PubMed
-
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996. April 4;380(6573):435–439. doi: 10.1038/380435a0 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

