Inhibition of interleukin-6 decreases atrogene expression and ameliorates tail suspension-induced skeletal muscle atrophy
- PMID: 29351340
- PMCID: PMC5774788
- DOI: 10.1371/journal.pone.0191318
Inhibition of interleukin-6 decreases atrogene expression and ameliorates tail suspension-induced skeletal muscle atrophy
Abstract
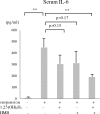
Background: Interleukin-6 (IL-6) is an inflammatory cytokine. Whether systemic IL-6 affects atrogene expression and disuse-induced skeletal muscle atrophy is unclear.
Methods: Tail-suspended mice were used as a disuse-induced muscle atrophy model. We administered anti-mouse IL-6 receptor antibody, beta-hydroxy-beta-methylbutyrate (HMB) and vitamin D to the mice and examined the effects on atrogene expression and muscle atrophy.
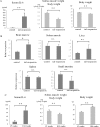
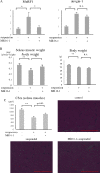
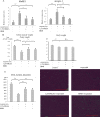
Results: Serum IL-6 levels were elevated in the mice. Inhibition of IL-6 receptor suppressed muscle RING finger 1 (MuRF1) expression and prevented muscle atrophy. HMB and vitamin D inhibited the serum IL-6 surge, downregulated the expression of MuRF1 and atrogin-1 in the soleus muscle, and ameliorated atrophy in the mice.
Conclusion: Systemic IL-6 affects MuRF1 expression and disuse-induced muscle atrophy.
Conflict of interest statement
Figures

References
-
- Foletta VC, White LJ, Larsen AE, Leger B, Russell AP. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflugers Arch. 2011;461:325–35. doi: 10.1007/s00424-010-0919-9 - DOI - PubMed
-
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034 - DOI - PMC - PubMed
-
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. doi: 10.1126/science.1065874 - DOI - PubMed
-
- Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–5. doi: 10.1073/pnas.251541198 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

