CYR61/CCN1 Regulates Sclerostin Levels and Bone Maintenance
- PMID: 29351359
- PMCID: PMC6002906
- DOI: 10.1002/jbmr.3394
CYR61/CCN1 Regulates Sclerostin Levels and Bone Maintenance
Abstract
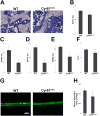
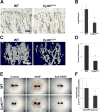
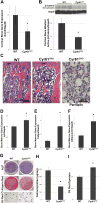
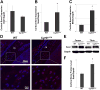
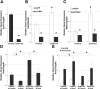
CYR61/CCN1 is a matricellular protein that resides in the extracellular matrix, but serves regulatory rather than structural roles. CYR61/CCN1 is found in mineralized tissues and has been shown to influence bone healing in vivo and osteogenic differentiation in vitro. In this study we generated Cyr61 bone-specific knockout mice to examine the physiological role of CYR61/CCN1 in bone development and maintenance in vivo. Extensive analysis of Cyr61 conditional knockout mice showed a significant decrease in both trabecular and cortical bone mass as compared to WT littermates. Our data suggest that CYR61/CCN1 exerts its effects on mature osteoblast/osteocyte function to modulate bone mass. Specifically, changes were observed in osteocyte/osteoblast expression of RankL, VegfA, and Sost. The increase in RankL expression was correlated with a significant increase in osteoclast number; decreased VegfA expression was correlated with a significant decrease in bone vasculature; increased Sost expression was associated with decreased Wnt signaling, as revealed by decreased Axin2 expression and increased adiposity in the bone marrow. Although the decreased number of vascular elements in bone likely contributes to the low bone mass phenotype in Cyr61 conditional knockout mice, this cannot explain the observed increase in osteoclasts and the decrease in Wnt signaling. We conducted in vitro assays using UMR-106 osteosarcoma cells to explore the role CYR61/CCN1 plays in modulating Sost mRNA and protein expression in osteocytes and osteoblasts. Overexpression of CYR61/CCN1 can suppress Sost expression in both control and Cyr61 knockout cells, and blocking Sost with siRNA can rescue Wnt responsiveness in Cyr61 knockout cells in vitro. Overall, our data suggest that CYR61/CCN1 modulates mature osteoblast and osteocyte function to regulate bone mass through angiogenic effects as well as by modulating Wnt signaling, at least in part through the Wnt antagonist Sost. © 2018 American Society for Bone and Mineral Research.
Keywords: ANIMAL MODELS; BONE MODELING AND REMODELING; CELL/TISSUE SIGNALING; CELLS OF BONE; GENETIC ANIMAL MODELS; MOLECULAR PATHWAYS; OSTEOCYTES; PARACRINE PATHWAYS; REMODELING; WNT/BETA-CATENIN/LRPS.
© 2018 American Society for Bone and Mineral Research.
Figures


Similar articles
-
CCN1/Cyr61 Is Required in Osteoblasts for Responsiveness to the Anabolic Activity of PTH.J Bone Miner Res. 2020 Nov;35(11):2289-2300. doi: 10.1002/jbmr.4128. Epub 2020 Aug 17. J Bone Miner Res. 2020. PMID: 32634285 Free PMC article.
-
Targeted disruption of BMP signaling through type IA receptor (BMPR1A) in osteocyte suppresses SOST and RANKL, leading to dramatic increase in bone mass, bone mineral density and mechanical strength.Bone. 2016 Oct;91:53-63. doi: 10.1016/j.bone.2016.07.002. Epub 2016 Jul 8. Bone. 2016. PMID: 27402532
-
Osteocyte network; a negative regulatory system for bone mass augmented by the induction of Rankl in osteoblasts and Sost in osteocytes at unloading.PLoS One. 2012;7(6):e40143. doi: 10.1371/journal.pone.0040143. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768243 Free PMC article.
-
Osteocyte-Mediated Translation of Mechanical Stimuli to Cellular Signaling and Its Role in Bone and Non-bone-Related Clinical Complications.Curr Osteoporos Rep. 2020 Feb;18(1):67-80. doi: 10.1007/s11914-020-00564-9. Curr Osteoporos Rep. 2020. PMID: 31953640 Review.
-
[Space flight/bedrest immobilization and bone. Osteocyte as a sensor of mechanical stress and Wnt signal].Clin Calcium. 2012 Dec;22(12):1829-35. Clin Calcium. 2012. PMID: 23187075 Review. Japanese.
Cited by
-
YAP and TAZ Mediate Osteocyte Perilacunar/Canalicular Remodeling.J Bone Miner Res. 2020 Jan;35(1):196-210. doi: 10.1002/jbmr.3876. Epub 2019 Oct 14. J Bone Miner Res. 2020. PMID: 31610061 Free PMC article.
-
Decrease of Cellular Communication Network Factor 1 (CCN1) Attenuates PTZ-Kindled Epilepsy in Mice.Cell Mol Neurobiol. 2023 Nov;43(8):4279-4293. doi: 10.1007/s10571-023-01420-x. Epub 2023 Oct 21. Cell Mol Neurobiol. 2023. PMID: 37864627 Free PMC article.
-
Hypoxia-initiated Cysteine-rich protein 61 secretion promotes chemoresistance of acute B lymphoblastic leukemia cells.Am J Cancer Res. 2024 Jul 15;14(7):3388-3403. doi: 10.62347/CKMT4065. eCollection 2024. Am J Cancer Res. 2024. PMID: 39113880 Free PMC article.
-
The regulation and functions of the matricellular CCN proteins induced by shear stress.J Cell Commun Signal. 2023 Jun;17(2):361-370. doi: 10.1007/s12079-023-00760-z. Epub 2023 May 16. J Cell Commun Signal. 2023. PMID: 37191841 Free PMC article. Review.
-
Osteogenic Differentiation of Mesenchymal Stem Cells Induced by Geometric Mechanotransductive 3D-Printed Poly-(L)-Lactic Acid Matrices.Int J Mol Sci. 2025 Aug 2;26(15):7494. doi: 10.3390/ijms26157494. Int J Mol Sci. 2025. PMID: 40806620 Free PMC article.
References
-
- Parisi MS, Gazzerro E, Rydziel S, Canalis E. Expression and regulation of CCN genes in murine osteoblasts. Bone. 2006;38(5):671–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

