Metabolic reprogramming in the pathogenesis of chronic lung diseases, including BPD, COPD, and pulmonary fibrosis
- PMID: 29351437
- PMCID: PMC5966782
- DOI: 10.1152/ajplung.00521.2017
Metabolic reprogramming in the pathogenesis of chronic lung diseases, including BPD, COPD, and pulmonary fibrosis
Abstract
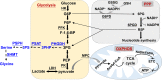
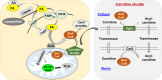
The metabolism of nutrient substrates, including glucose, glutamine, and fatty acids, provides acetyl-CoA for the tricarboxylic acid cycle to generate energy, as well as metabolites for the biosynthesis of biomolecules, including nucleotides, proteins, and lipids. It has been shown that metabolism of glucose, fatty acid, and glutamine plays important roles in modulating cellular proliferation, differentiation, apoptosis, autophagy, senescence, and inflammatory responses. All of these cellular processes contribute to the pathogenesis of chronic lung diseases, including bronchopulmonary dysplasia, chronic obstructive pulmonary disease, and pulmonary fibrosis. Recent studies demonstrate that metabolic reprogramming occurs in patients with and animal models of chronic lung diseases, suggesting that metabolic dysregulation may participate in the pathogenesis and progression of these diseases. In this review, we briefly discuss the catabolic pathways for glucose, glutamine, and fatty acids, and focus on how metabolic reprogramming of these pathways impacts cellular functions and leads to the development of these chronic lung diseases. We also highlight how targeting metabolic pathways can be utilized in the prevention and treatment of these diseases.
Keywords: bronchopulmonary dysplasia; chronic obstructive pulmonary disease; metabolic flexibility; metabolic reprogramming; pulmonary fibrosis.
Figures

References
-
- Agarwal AR, Zhao L, Sancheti H, Sundar IK, Rahman I, Cadenas E. Short-term cigarette smoke exposure induces reversible changes in energy metabolism and cellular redox status independent of inflammatory responses in mouse lungs. Am J Physiol Lung Cell Mol Physiol 303: L889–L898, 2012. doi: 10.1152/ajplung.00219.2012. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

