Activated human T lymphocytes inhibit TGFβ-induced fibroblast to myofibroblast differentiation via prostaglandins D2 and E2
- PMID: 29351444
- PMCID: PMC5966783
- DOI: 10.1152/ajplung.00565.2016
Activated human T lymphocytes inhibit TGFβ-induced fibroblast to myofibroblast differentiation via prostaglandins D2 and E2
Abstract
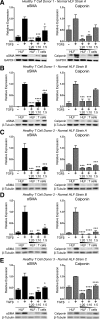
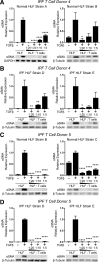
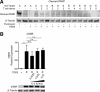
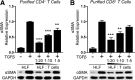
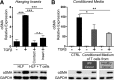
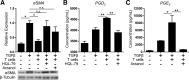
In pulmonary fibrosis (PF), fibroblasts and myofibroblasts proliferate and deposit excessive extracellular matrix in the interstitium, impairing normal lung function. Because most forms of PF have a poor prognosis and limited treatment options, PF represents an urgent unmet need for novel, effective therapeutics. Although the role of immune cells in lung fibrosis is unclear, recent studies suggest that T lymphocyte (T cell) activation may be impaired in PF patients. Furthermore, we have previously shown that activated T cells can produce prostaglandins with anti-scarring potential. Here, we test the hypothesis that activated T cells directly inhibit myofibroblast differentiation using a coculture system. Coculture with activated primary blood-derived T cells, from both healthy human donors and PF patients, inhibited transforming growth factor β-induced myofibroblast differentiation in primary human lung fibroblasts isolated from either normal or PF lung tissue. Coculture supernatants contained anti-fibrotic prostaglandins D2 and E2, and the inhibitory effect of coculture on myofibroblast differentiation was largely reversed when prostaglandin production was abrogated either by resting the T cells before coculture or via specific pharmacological inhibitors. Moreover, coculture conditions induced COX-2 in HLFs but not in T cells, suggesting that T cells deliver an activating signal to HLFs, which in turn produce anti-fibrotic prostaglandins. We show for the first time that coculture with activated primary human T lymphocytes strongly inhibits myofibroblast differentiation, revealing a novel cell-to-cell communication network with therapeutic implications for fibrotic lung diseases.
Figures





Similar articles
-
Normal Human Lung Epithelial Cells Inhibit Transforming Growth Factor-β Induced Myofibroblast Differentiation via Prostaglandin E2.PLoS One. 2015 Aug 6;10(8):e0135266. doi: 10.1371/journal.pone.0135266. eCollection 2015. PLoS One. 2015. PMID: 26248335 Free PMC article.
-
Activated Human Lung Fibroblasts Produce Extracellular Vesicles with Antifibrotic Prostaglandins.Am J Respir Cell Mol Biol. 2019 Mar;60(3):269-278. doi: 10.1165/rcmb.2017-0248OC. Am J Respir Cell Mol Biol. 2019. PMID: 30265126 Free PMC article.
-
Gal-1-mediated cytochrome p450 activation promotes fibroblast into myofibroblast differentiation in pulmonary fibrosis.Int Immunopharmacol. 2024 Nov 15;141:112920. doi: 10.1016/j.intimp.2024.112920. Epub 2024 Aug 12. Int Immunopharmacol. 2024. PMID: 39137631
-
TGFβ signaling and the control of myofibroblast differentiation: Implications for chronic inflammatory disorders.J Cell Physiol. 2018 Jan;233(1):98-106. doi: 10.1002/jcp.25879. Epub 2017 May 15. J Cell Physiol. 2018. PMID: 28247933 Review.
-
Cellular players in lung fibrosis.Curr Pharm Des. 2012;18(27):4093-102. doi: 10.2174/138161212802430396. Curr Pharm Des. 2012. PMID: 22630084 Review.
Cited by
-
Prostaglandin E2 inhibits profibrotic function of human pulmonary fibroblasts by disrupting Ca2+ signaling.Am J Physiol Lung Cell Mol Physiol. 2019 May 1;316(5):L810-L821. doi: 10.1152/ajplung.00403.2018. Epub 2019 Feb 13. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30758990 Free PMC article.
-
ADAM17/PTGS2 Facilitates Pulmonary Fibrosis by Regulating Ferroptosis.J Cell Mol Med. 2025 Mar;29(5):e70466. doi: 10.1111/jcmm.70466. J Cell Mol Med. 2025. PMID: 40077919 Free PMC article.
-
Catalpol Protects Against Pulmonary Fibrosis Through Inhibiting TGF-β1/Smad3 and Wnt/β-Catenin Signaling Pathways.Front Pharmacol. 2021 Jan 29;11:594139. doi: 10.3389/fphar.2020.594139. eCollection 2020. Front Pharmacol. 2021. PMID: 33584272 Free PMC article.
-
In vitro co-culture studies and the crucial role of fibroblast-immune cell crosstalk in IPF pathogenesis.Respir Res. 2023 Nov 27;24(1):298. doi: 10.1186/s12931-023-02608-x. Respir Res. 2023. PMID: 38012580 Free PMC article. Review.
-
Extracellular Lipids in the Lung and Their Role in Pulmonary Fibrosis.Cells. 2022 Apr 3;11(7):1209. doi: 10.3390/cells11071209. Cells. 2022. PMID: 35406772 Free PMC article. Review.
References
-
- Adegunsoye A, Hrusch CL, Bonham CA, Jaffery MR, Blaine KM, Sullivan M, Churpek MM, Strek ME, Noth I, Sperling AI. Skewed Lung CCR4 to CCR6 CD4+T Cell Ratio in Idiopathic Pulmonary Fibrosis Is Associated with Pulmonary Function. Front Immunol 7: 516, 2016. doi:10.3389/fimmu.2016.00516. - DOI - PMC - PubMed
-
- Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L1146–L1153, 2005. doi:10.1152/ajplung.00383.2004. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

