From ionic to cellular variability in human atrial myocytes: an integrative computational and experimental study
- PMID: 29351467
- PMCID: PMC6008144
- DOI: 10.1152/ajpheart.00477.2017
From ionic to cellular variability in human atrial myocytes: an integrative computational and experimental study
Abstract
Variability refers to differences in physiological function between individuals, which may translate into different disease susceptibility and treatment efficacy. Experiments in human cardiomyocytes face wide variability and restricted tissue access; under these conditions, computational models are a useful complementary tool. We conducted a computational and experimental investigation in cardiomyocytes isolated from samples of the right atrial appendage of patients undergoing cardiac surgery to evaluate the impact of variability in action potentials (APs) and subcellular ionic densities on Ca2+ transient dynamics. Results showed that 1) variability in APs and ionic densities is large, even within an apparently homogenous patient cohort, and translates into ±100% variation in ionic conductances; 2) experimentally calibrated populations of models with wide variations in ionic densities yield APs overlapping with those obtained experimentally, even if AP characteristics of the original generic model differed significantly from experimental APs; 3) model calibration with AP recordings restricts the variability in ionic densities affecting upstroke and resting potential, but redundancy in repolarization currents admits substantial variability in ionic densities; and 4) model populations constrained with experimental APs and ionic densities exhibit three Ca2+ transient phenotypes, differing in intracellular Ca2+ handling and Na+/Ca2+ membrane extrusion. These findings advance our understanding of the impact of variability in human atrial electrophysiology. NEW & NOTEWORTHY Variability in human atrial electrophysiology is investigated by integrating for the first time cellular-level and ion channel recordings in computational electrophysiological models. Ion channel calibration restricts current densities but not cellular phenotypic variability. Reduced Na+/Ca2+ exchanger is identified as a primary mechanism underlying diastolic Ca2+ fluctuations in human atrial myocytes.
Keywords: action potential; population of models.
Figures






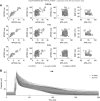


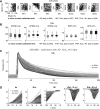



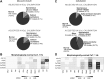


References
-
- Abi-Gerges N, Small BG, Lawrence CL, Hammond TG, Valentin J-P, Pollard CE. Gender differences in the slow delayed (IKs) but not in inward (IK1) rectifier K+ currents of canine Purkinje fibre cardiac action potential: key roles for IKs, beta-adrenoceptor stimulation, pacing rate and gender. Br J Pharmacol 147: 653–660, 2006. doi:10.1038/sj.bjp.0706491. - DOI - PMC - PubMed
-
- Britton OJ, Bueno-Orovio A, Van Ammel K, Lu HR, Towart R, Gallacher DJ, Rodriguez B. Experimentally calibrated population of models predicts and explains intersubject variability in cardiac cellular electrophysiology. Proc Natl Acad Sci USA 110: E2098–E2105, 2013. doi:10.1073/pnas.1304382110. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

