Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa
- PMID: 29351513
- PMCID: PMC5866360
- DOI: 10.1152/physrev.00039.2016
Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa
Abstract
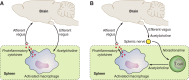
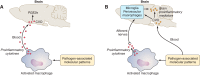
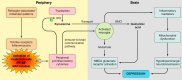
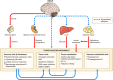
Because of the compartmentalization of disciplines that shaped the academic landscape of biology and biomedical sciences in the past, physiological systems have long been studied in isolation from each other. This has particularly been the case for the immune system. As a consequence of its ties with pathology and microbiology, immunology as a discipline has largely grown independently of physiology. Accordingly, it has taken a long time for immunologists to accept the concept that the immune system is not self-regulated but functions in close association with the nervous system. These associations are present at different levels of organization. At the local level, there is clear evidence for the production and use of immune factors by the central nervous system and for the production and use of neuroendocrine mediators by the immune system. Short-range interactions between immune cells and peripheral nerve endings innervating immune organs allow the immune system to recruit local neuronal elements for fine tuning of the immune response. Reciprocally, immune cells and mediators play a regulatory role in the nervous system and participate in the elimination and plasticity of synapses during development as well as in synaptic plasticity at adulthood. At the whole organism level, long-range interactions between immune cells and the central nervous system allow the immune system to engage the rest of the body in the fight against infection from pathogenic microorganisms and permit the nervous system to regulate immune functioning. Alterations in communication pathways between the immune system and the nervous system can account for many pathological conditions that were initially attributed to strict organ dysfunction. This applies in particular to psychiatric disorders and several immune-mediated diseases. This review will show how our understanding of this balance between long-range and short-range interactions between the immune system and the central nervous system has evolved over time, since the first demonstrations of immune influences on brain functions. The necessary complementarity of these two modes of communication will then be discussed. Finally, a few examples will illustrate how dysfunction in these communication pathways results in what was formerly considered in psychiatry and immunology to be strict organ pathologies.
Figures

References
-
- Ader R. Historical perspectives on psychoneuroimmunology. In: Psychoneuroimmunology, Stress, and Infection, edited by Friedman H, Klein TW, Friedman AL. Boca Raton, FL: CRC, 1995, p. 1–21.
-
- Armaiz-Pena GN, Allen JK, Cruz A, Stone RL, Nick AM, Lin YG, Han LY, Mangala LS, Villares GJ, Vivas-Mejia P, Rodriguez-Aguayo C, Nagaraja AS, Gharpure KM, Wu Z, English RD, Soman KV, Shahzad MM, Zigler M, Deavers MT, Zien A, Soldatos TG, Jackson DB, Wiktorowicz JE, Torres-Lugo M, Young T, De Geest K, Gallick GE, Bar-Eli M, Lopez-Berestein G, Cole SW, Lopez GE, Lutgendorf SK, Sood AK. Src activation by β-adrenoreceptors is a key switch for tumour metastasis. Nat Commun 4: 1403, 2013. doi: 10.1038/ncomms2413. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

