Mitochondrial Uncoupling Proteins: Subtle Regulators of Cellular Redox Signaling
- PMID: 29351723
- PMCID: PMC6071544
- DOI: 10.1089/ars.2017.7225
Mitochondrial Uncoupling Proteins: Subtle Regulators of Cellular Redox Signaling
Abstract
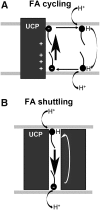
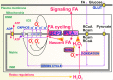
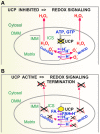
Significance: Mitochondria are the energetic, metabolic, redox, and information signaling centers of the cell. Substrate pressure, mitochondrial network dynamics, and cristae morphology state are integrated by the protonmotive force Δp or its potential component, ΔΨ, which are attenuated by proton backflux into the matrix, termed uncoupling. The mitochondrial uncoupling proteins (UCP1-5) play an eminent role in the regulation of each of the mentioned aspects, being involved in numerous physiological events including redox signaling. Recent Advances: UCP2 structure, including purine nucleotide and fatty acid (FA) binding sites, strongly support the FA cycling mechanism: UCP2 expels FA anions, whereas uncoupling is achieved by the membrane backflux of protonated FA. Nascent FAs, cleaved by phospholipases, are preferential. The resulting Δp dissipation decreases superoxide formation dependent on Δp. UCP-mediated antioxidant protection and its impairment are expected to play a major role in cell physiology and pathology. Moreover, UCP2-mediated aspartate, oxaloacetate, and malate antiport with phosphate is expected to alter metabolism of cancer cells.
Critical issues: A wide range of UCP antioxidant effects and participations in redox signaling have been reported; however, mechanisms of UCP activation are still debated. Switching off/on the UCP2 protonophoretic function might serve as redox signaling either by employing/releasing the extra capacity of cell antioxidant systems or by directly increasing/decreasing mitochondrial superoxide sources. Rapid UCP2 degradation, FA levels, elevation of purine nucleotides, decreased Mg2+, or increased pyruvate accumulation may initiate UCP-mediated redox signaling.
Future directions: Issues such as UCP2 participation in glucose sensing, neuronal (synaptic) function, and immune cell activation should be elucidated. Antioxid. Redox Signal. 29, 667-714.
Keywords: UCP2; anion transport; attenuation of superoxide formation; fatty acid cycling; mitochondrial uncoupling proteins; redox signaling.
Figures













Similar articles
-
H₂O₂-Activated Mitochondrial Phospholipase iPLA₂γ Prevents Lipotoxic Oxidative Stress in Synergy with UCP2, Amplifies Signaling via G-Protein-Coupled Receptor GPR40, and Regulates Insulin Secretion in Pancreatic β-Cells.Antioxid Redox Signal. 2015 Oct 20;23(12):958-72. doi: 10.1089/ars.2014.6195. Epub 2015 May 21. Antioxid Redox Signal. 2015. PMID: 25925080 Free PMC article.
-
Antioxidant activity by a synergy of redox-sensitive mitochondrial phospholipase A2 and uncoupling protein-2 in lung and spleen.Int J Biochem Cell Biol. 2013 Apr;45(4):816-25. doi: 10.1016/j.biocel.2013.01.010. Epub 2013 Jan 24. Int J Biochem Cell Biol. 2013. PMID: 23354121
-
Uncoupling mechanism and redox regulation of mitochondrial uncoupling protein 1 (UCP1).Biochim Biophys Acta Bioenerg. 2019 Mar 1;1860(3):259-269. doi: 10.1016/j.bbabio.2018.11.007. Epub 2018 Nov 8. Biochim Biophys Acta Bioenerg. 2019. PMID: 30414927 Review.
-
Antioxidant and regulatory role of mitochondrial uncoupling protein UCP2 in pancreatic beta-cells.Physiol Res. 2014;63(Suppl 1):S73-91. doi: 10.33549/physiolres.932633. Physiol Res. 2014. PMID: 24564667 Review.
-
Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants.J Biol Chem. 2002 Dec 6;277(49):47129-35. doi: 10.1074/jbc.M208262200. Epub 2002 Oct 7. J Biol Chem. 2002. PMID: 12372827
Cited by
-
Contribution of Oxidative Stress and Impaired Biogenesis of Pancreatic β-Cells to Type 2 Diabetes.Antioxid Redox Signal. 2019 Oct 1;31(10):722-751. doi: 10.1089/ars.2018.7656. Epub 2019 Jan 23. Antioxid Redox Signal. 2019. PMID: 30450940 Free PMC article.
-
NADPH oxidase 4 is dispensable for skin myofibroblast differentiation and wound healing.Redox Biol. 2023 Apr;60:102609. doi: 10.1016/j.redox.2023.102609. Epub 2023 Jan 13. Redox Biol. 2023. PMID: 36708644 Free PMC article.
-
Melatonin Alleviates Acute Kidney Injury by Inhibiting NRF2/Slc7a11 Axis-Mediated Ferroptosis.Oxid Med Cell Longev. 2022 Aug 8;2022:4776243. doi: 10.1155/2022/4776243. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35979396 Free PMC article.
-
A Population of M2 Macrophages Associated With Bone Formation.Front Immunol. 2021 Oct 12;12:686769. doi: 10.3389/fimmu.2021.686769. eCollection 2021. Front Immunol. 2021. PMID: 34712222 Free PMC article.
-
Uncoupling Proteins as Therapeutic Targets for Neurodegenerative Diseases.Int J Mol Sci. 2022 May 18;23(10):5672. doi: 10.3390/ijms23105672. Int J Mol Sci. 2022. PMID: 35628482 Free PMC article. Review.
References
-
- Abrahams JP, Leslie AGW, Lutter R, and Walker JE. Structure at 2.8 Â resolution of F1-ATPase from bovine heart mitochondria. Nature 370: 621–628, 1994 - PubMed
-
- Adams AE, Carroll AM, Fallon PG, and Porter RK. Mitochondrial uncoupling protein 1 expression in thymocytes. Biochim Biophys Acta 1777: 772–776, 2008 - PubMed
-
- Adams AE, Hanrahan O, Nolan DN, Voorheis HP, Fallon P, and Porter RK. Images of mitochondrial UCP 1 in mouse thymocytes using confocal microscopy. Biochim Biophys Acta 1777: 115–117, 2008 - PubMed
-
- Adams AE, Kelly OM, and Porter RK. Absence of mitochondrial uncoupling protein 1 affects apoptosis in thymocytes, thymocyte/T-cell profile and peripheral T-cell number. Biochim Biophys Acta 1797: 807–816, 2010 - PubMed
-
- Affourtit C. and Brand MD. Uncoupling protein-2 contributes significantly to high mitochondrial proton leak in INS-1E insulinoma cells and attenuates glucose-stimulated insulin secretion. Biochem J 409: 199–204, 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
